Respirator and medical masks are being advocated as the most basic infection control measure. More specifically the N95 FFRs (filtering facepiece respirators) comparable masks in other countries (e.g., FFP2, KN95, DS/DL2, KF94) are the leading personal protective equipment (PPE) recommended by the Centers for Disease Control and Prevention (CDC) and are therefore, playing a crucial role in maintaining the health of medical staff treating corona patients worldwide, as well as for public health needs.
The increased demand had led to a shortage in the supply of sterile N95 FFRs, and to governments and medical institutions seeking for a safe, easy and accessible sterilization solution.

What is hydrogen peroxide sterilization?
Low temperature sterilization is a sterilization process best used for heat-sensitive devices that may be damaged by the conditions of a steam sterilization cycle.
Hydrogen peroxide (H2O2) sterilization, also known as hydrogen peroxide gas sterilization, used in plasma sterilizers, is a low temperature sterilization process commonly used to sterilize heat-sensitive devices.
A hydrogen peroxide sterilization process involves filling the sterilizer chamber with H2O2 vapor. Once the sterilization cycle is complete, the vapor is vacuumed from the chamber and converted to water and oxygen.
Examples of current clinical validations of mask sterilization
There are a few companies and clinical evidence validating the sterilization of FFR masks using hydrogen peroxide sterilization.
The U.S. Food and Drug (FDA) recently granted the Battelle Critical Care Decontamination System (CCDS Columbus, OH, USA) Emergency Use Authorization (EUA) for large-scale and repeat (up to 20 cycles) PPE decontamination of N95 masks. The vapor phase hydrogen peroxide (VPHP) method exposes protective equipment to concentrated hydrogen peroxide vapors, in a 2.5-hour treatment cycle, destroying bacteria, viruses and other contaminants, including the novel coronoavirus SARS-CoV-2. Feasibility testing of the system demonstrated a 6-log reduction in G. stearothermophilus counts, without jeopardizing N95 respirator filter performance over multiple treatment cycles1.
The Dutch National Institute for Public Health and Environment (RIVM) reported on similar results following exploratory FFP2 face mask reprocessing using hydrogen peroxide sterilization2. Unused FFP2 masks were
reprocessed in up to four VPHP treatment cycles, which was previously shown to inactivate viruses. Reprocessed masks passed a standard fit test, with masks showing retained barrier efficiency, for up to two treatment cycles. Preliminary data suggest that the method is applicable for cellulose-containing masks as well.
Tuttnauer Study
Tuttnauer, a world-leading company providing infection control solutions since 1925, manufactures a similar VPHP platform (also called a low temperature H2O2 plasma sterilizer), which has been tested on the valved 3M Welding Fume Respirator 9925. The target of the following reported study was to demonstrate the ability to sterilize FFRs by low-temperature sterilization without affecting their performance.
Material & Methods
N95 FFR sterilization process
Six (6) N95 FFR masks, type - 3M™ Welding Fume Respirator, FFP2, Valved, 9925, were placed in a Tuttnauer 160-liter PlazMax chamber, as demonstrated in Pic. 1. The masks where divided into 3 groups. One (1) mask served as a control, i.e., did not undergo any sterilization cycle. Three (3) masks were subjecyted to 3 sterilization cycles. and two masks were exposed to 5 sterilization cycles. sterilizer was then run on "Normal" program.
Vaporized hydrogen peroxide (VHP) sterilization process
The vaporized hydrogen peroxide sterilization process is constructed of four (4) main stages:
1. Sterilization chamber pressure is reduced to a very high vacuum
2. Liquid H2O2 is converted into vapor
3. Under high vacuum, vapors fill the chamber, contacting all surfaces
4. After sterilization, the vapor is vacuumed from the chamber and converted into water and oxygen
Diagram 1 (below) presents the four stages of VHP sterilization process carried out by the PlazMax VHP sterilizer in the presence of the FFR masks.
The process is carried out under a constant temperature of 55°C.
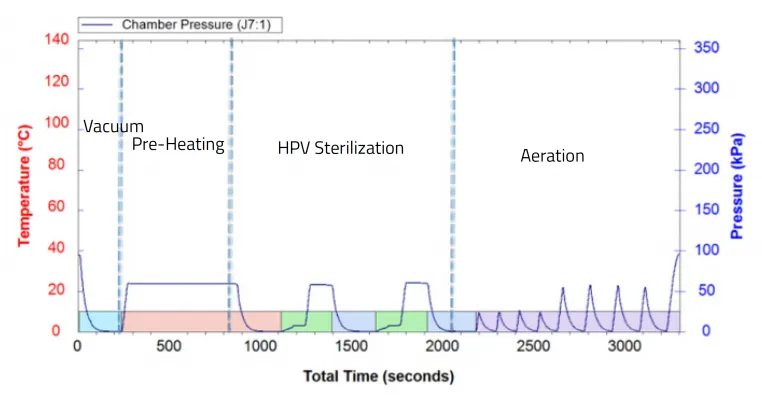
Air flow measurements through sterilized FFR masks
The air flow through the sterilized and non-sterilized FFR masks was tested using the FX 3300 Air Permeability Tester III, by TEXTEST Instruments, as presented below in Pic. 2, following the WSP 70.1 standard for non-woven applications.
The system measures air flow under constant air pressure.
Results are measured as ft3/min - cfm.
Testing conditions:
• Tested area: 20 cm2
• Pressure: 125 Pa
The masks were cut into half and around the mono-directional filter, in order to fit into the test equipment.

Results & Discussion
Table 1. below demonstrates the air flow (cfm) through the tested masks.
| Mask # | Air Flow [cfm] - No sterilization | Air Flow [cfm] - 3 sterilization cycles | Air Flow [cfm] - 5 sterilization cycles |
| 1 | 39.5 | 37.9 | 37.6 |
| 39.6 | 35 | 30.2 | |
| 2 | 36.6 | 48 | |
| 30.6 | 51.2 | ||
| Average air flow | 39.55 | 35.03 | 41.75 |
| Standard deviation | 0.07 | 3.18 | 9.64 |
| [%] Air flow change | -11% | +6% |
It can be seen that average air flow was slightly reduced after 3 sterilization cycles (11% reduction in air flow) while after 5 sterilization cycles the air flow remained more or less the same (6% increase). Although the sample size was small, and taking into consideration the measuring unit limitations and inherent deviations in the product, it can be seen that there are no significant changes in the air flow through the masks. That can demonstrate no effect on the non-woven material.
Tuttnauer Validation Summary
A study, targeted to demonstrate the ability to sterilize FFRs by low temperature sterilization, with TuttnauerHPV PlazMax, without affecting their performance, has been conducted.
The results, although based on a small test group, showed no significant changes in the air flow through the masks even after 5 sterilization cycles.
Tuttnauer Recommended Cycle
For easy sterilization and reuse of the single use masks (including N95 FFRs filtering face piece respirators) simply choose the “normal” cycle program in Tuttnauer PlazMax sterilizer.
Take the following steps:
- Switch on the machine
- The first screen to appear is the main home screen (verify that the door is open to allow choosing the right program)
Note: If the machine is already pre-set for “Normal” program, place your masks in the chamber and press start to run the cycle.
- Total mask capacity per load is 120. To maximize your load, masks can be placed on top of each other
- If local policy calls for a single packaging of the masks, please place the packaged masks on top of each other. Make sure not to exceed the basket’s dimensions.
- Normal cycle run for 45 min. Thereafter the masks are sterilize and ready to be used again
- There is no need for extra aeration post-cycle. A new cycle can be immediately operated
Commercially Used References
This sterilization technique is commercially approved an used around the globe.
Coronavirus In Ohio: Battelle Pioneers Technology To Clean And Reuse PPE
https://radio.wosu.org/post/coronavirus-ohio-battelle-pioneers-technology-clean-and-reuse-ppe#stream/0
FDA lifts restrictions on Ohio-based Battelle's mask-sterilizing technology amid coronavirus shortages
https://www.usatoday.com/story/news/nation/2020/03/29/coronavirus-fda-eases-restrictions-masksterilization-technology/2936670001/
Other References
1) Ref: N95 FFR Decontamination for Reuse Final Report July 22, 2016
2) Ref: Hergebruik mondkapjes March 18, 2020