Bottom line? Filtration is a great option for sterilizing heat-sensitive liquids, which cannot be autoclaved or sterilized by other sterilization methods. In this blog post, we will explore the ways in which different types of filtration systems work, as well as the practical advantages and disadvantages of using them in a laboratory setting.
What Is Filtration and How Does It work?
Filtration is different from other sterilization methods. Wikipedia defines sterilization as a process that eliminates (removes) or kills (deactivates) all forms of life and other biological agents. Up until now all the sterilization methods we covered deactivate or kill bacteria and viruses. Filtration is the first and only sterilization method that eliminates bacteria by separating the microorganisms from the sterilized medium, but unlike other sterilization methods, it doesn’t kill or stop the bacteria's ability to reproduce. The way it works is actually very simple. You’re probably familiar with water filters found in an office or at home, or a coffee percolator, all of which use the same basic mechanism of filtration.
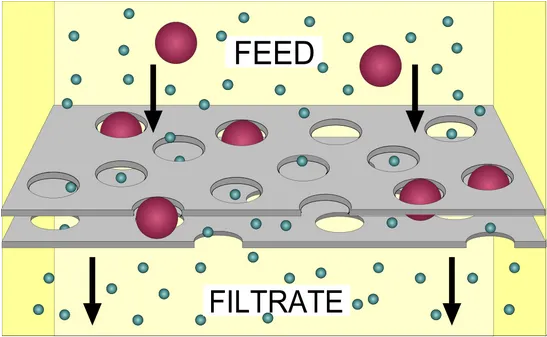
Filtration uses membranous filters that have tiny pores that let the liquid pass through but prevent bigger particles such as bacteria to pass through the filter. Therefore, the smaller the pore, the more likely the filter is to stop more things from going through it. If the pores of a filter, which is designed to remove a microbe, which is a microscopic organism, are small enough, they should be able to stop all living things from passing through.
Filtration of Liquids in the Lab
In laboratories, liquids are filtered through microbial filters to remove any microbes present. It is an effective method of sterilization for heat sensitive liquids. There are four types of filters:
- Membrane filters are thin filters that are made of cellulose. They can be used for sterilization during injection by placing the membrane between the syringe and the needle.
- Seitz filters are usually made of asbestos. They are pad-like and thicker than membrane filters.
- Sintered glass filters are an alternative type of filter that are made of glass and hence do not absorb liquids during filtration.
- Candle filters are made of clay-like mud. This special mud has tiny pores made by algae. The microbes get stuck during their travel through the pores.
Filtration Techniques
There is more than one filtration technique. Reverse Osmosis is used in home filtration systems. Other common methods are nano-filtration, ultra-filtration, micro-filtration and particle filtration. We will not discuss these different techniques since they are beyond the scope of this article, but it’s important to know that are several techniques and each technique is suitable for a specific application. Here’s a chart that shows the different uses of these filters:

To sum up the first section of the article watch this video that demonstrates filtration in the lab:
Advantages of Filtration
- Relatively inexpensive, except for those with the smallest pore sizes
- Filters do not clog easily
- Suitable for heat sensitive liquids as filters do not use heat
- They can filter large volumes of fluid reasonably fast
Disadvantages of Filtration
- Filters can only work on liquids and gasses
- Autoclaving is usually cheaper than filtration since filters are expensive to replace, especially nano-filters
- Glass filters are very brittle and can break easily
- Membrane filters rupture easily
- The solution in Sietz Filters might get absorbed by the filter pad itself
- Clogging may occur
- Long process
Conclusion
Let’s recap. We explored that filters are a physical method that separates microbes like bacteria from liquids. Then we explained common uses of filtration in the lab. We are thankful for filters as they play an important role in laboratory virus research since Charles Chamberlain first used them in the late 19th century. But, if possible, it’s always recommended to sterilize a solution with an autoclave and use filtration only when autoclaving is impossible. The rule is that any solution used in the lab should be sterilized.
What type of filtration do you use in your lab? We’d love to hear your take on filtration in the comments section below.