In this post, we will focus on one specific application in the world of sterilization and infection control: that of radiation’s ability to kill harmful microorganisms. In other words, radiation can be used to sterilize medical and scientific equipment. But before we can understand this application, let’s review a short history of radiation.

History
The history of sterilization by radiation dates back to 1895 when X-rays were first discovered by W.C. Roentgen. In 1896 Henri Becquerel observed radiation emitted from uranium. And in 1898 Marie and Pierre Curie discovered several radioactive elements and first used the term “radioactive.” Marie received the Nobel prize twice in two different scientific disciplines even though she suffered from a blood illness attributed to prolonged exposure to radiation. She later died from this illness, known as aplastic anemia, at the age of 66. The damaging effects of radiation were not known at the time and therefore no safety measures were taken. Curie carried test tubes containing radioactive isotopes in her pocket, and stored them in her desk drawer, remarking on the faint light that the substances gave off in the dark room.
In 1958 the first commercial food irradiation plant was installed at Stuttgart, Germany, which processed spices. In 1963 the first gamma irradiator was installed in the US for the processing and sterilization of medical devices. In 1964 the Atomic Energy of Canada commissioned the first industrial cobalt-60 sterilization facility to sterilize surgical suture. Today, 40–50% of disposable medical products manufactured in developed countries are radiation sterilized and there are over 200 gamma irradiators worldwide in operation for a variety of purposes.

Ionizing Radiation
Ionizing radiation is a type of short wavelength radiation that carries enough energy to free electrons from atoms or molecules, thereby ionizing them. This article focuses on gamma irradiation, which is most commonly used for sterilization purposes.
The bottom line of how this works is that the ionizing radiation produces disruptions in sub-atomic particles involved in the formation of the microorganism. Simply put, this radiation causes damage to the genetic material - the DNA or the RNA - of the organism’s cell. If the DNA or RNA of a microorganism is damaged, the cell will die. In other words, radiation damages the hard drive of a bacterium, causing it to shut down for good.
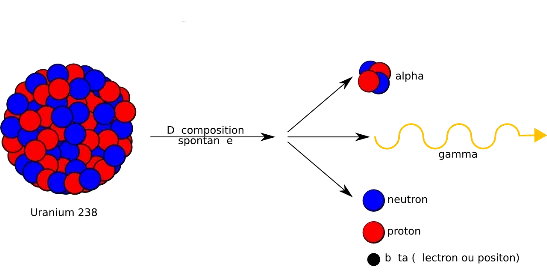
Decomposition and Gamma Rays
Let’s dive a little deeper in an attempt to explain how gamma ray radiation is produced. Every physical substance has natural energy levels, which can be increased or decreased artificially in the lab. Gamma radiation is created by the decomposition of an atom. Gamma radiation is a natural radiation that is emitted from an atom or a molecule when its energy level drops. To take an extreme example, when radioactive uranium decomposes it releases energy to such an extent that it’s known as an atom bomb explosion (fission).
So now we understand that in fact every substance that decomposes emits some electromagnetic radiation, but not every electromagnetic radiation is fit for sterilization. Some substances are too powerful, uranium and plutonium for example. They might sterilize, but they will also kill everything else in the environment. Not exactly the desired outcome. So if uranium and plutonium are not fit for sterilization, what is?
Cobalt: Not too Powerful, Not too weak
Gamma rays used for sterile processing are formed with the self disintegration of Cobalt-60 (60Co). Among thousands of gamma emitters only Cobalt-60 is indicated for sterilization processing. Cobalt-60 can be produced in a nuclear power reactor by the irradiation of 59Co (metal), with fast neutrons.
The radioactive isotope is formed by neutron capture as shown in the following equation:
27Co59+ n→ 27Co60
Cobalt-60 is manufactured specifically for the irradiation process and is housed in specially designed chambers operating under strict standards and regulations. The gamma irradiation sterilization process does not involve sufficient energy to cause the treated products to become radioactive; it will only harm the microorganisms on the products.

Will Cobalt-60 Live Life to the Fullest?
We all want to live our lives to the fullest, but when it comes to Cobalt-60, we are very satisfied with a half life. In order to understand gamma ray irradiation, we need to explain what the half-life of a radioactive substance is. It is the amount of time it takes for half of the atoms of the radioactive substance to decay. For example, if we start out with a 800,000 Curie of Cobalt-60, after 5.27 years 400,000 will remain. The amount of energy remaining after this period is not sufficient for sterilization; this is when the dose is returned to the production company, and they will replace it with a fresh new package of 800,000 Curie. This, of course, has a price tag, a very high one, well over $1 million!
Cold Process Sterilization
Currently, all industrial radiation processing facilities employ Cobalt-60 as the gamma radiation source. The reason why Cobalt-60 is the most suitable for radiation processing is because of the relatively high energy of their gamma rays and fairly long half-life which is 5.27 years.
A while ago we began the sterilization methods series with a discussion of methods that use heat and focused on autoclaving. We then continued to discuss non-heat methods: Ethylene oxide (EtO) and Formaldehyde that are used for sterilizing heat sensitive items. These two methods use a chemical agent to sterilize.
Just like EtO and formaldehyde, gamma irradiation is known as a ‘Cold Process,’ as the temperature of the processed product does not increase significantly. Therefore, it’s a suitable sterilization method for heat sensitive items. Gamma irradiation does not rely on humidity, temperature or pressure and can be applied to packaged goods.
Gamma irradiation is a physical/chemical means of sterilization, because it kills bacteria by breaking down bacterial DNA, inhibiting bacterial division. Energy of gamma rays passes through the equipment, disrupting the pathogens that cause contamination. These changes at the molecular level cause the death of contaminating organisms or render such organisms incapable of reproduction. The gamma irradiation process does not create residuals or impart radioactivity in the processed items. Complete penetration can be achieved depending on the thickness of the material.
Applications
Gamma sterilization is used to sterilize human tissue grafts: connective tissue allograft, such as bone, cartilage, tendons, ligaments, dura mater, skin, heart valves and corneas, which are widely used for reconstructive surgery in many clinical disciplines, including orthopedics, traumatology, neurosurgery, cardiac surgery, plastic surgery, laryngology, and ophthalmology.
Sterilization by radiation is also used for sterilization of plastic syringes, hypodermic needles, scalpels, surgical blades, adhesive dressings and thermolabile medicaments.
Other applications include: syringes, surgical gloves, gowns, masks, sticking plasters, dressings, ‘tetrapacks,’ bottle teats for premature babies, food packaging, raw materials for pharmaceuticals and cosmetics, and even wine corks.
Another common application of sterilization by Gamma irradiation is food. Food sterilization by gamma irradiation is the process of exposing food to ionizing radiation to destroy microorganisms, namely bacteria, or insects that might be present in the food.
Advantages
- Gamma rays have a high penetration power so materials can be sterilized after filling them in the final container
- The method is suitable for all types of materials such as dry, moist and even frozen items
- The method is considered to be reliable and can be accurately controlled
Disadvantages
- There is some risk involved since exposure to radiation may be harmful to workers
- It can produce undesirable changes in medicine such as color, solubility and texture of the product
- It can actually damage the material it’s meant to sterilize
- It’s expensive
Summary
Using radiation for sterilization is great if you need to sterilize products that are heat and moisture sensitive and cannot be sterilized in an autoclave. However using ionizing radiation for sterilization isn’t always practical. Other reasons that this method is less popular is that it is very expensive and requires a warehouse-like processing facility. It can be very dangerous if used improperly. After all, if it can kill bacteria it can also kill humans.
What’s your take? Let us know your thoughts about radiation sterilization in the comments below.
Dr. Mario Finkiel, Ph.D.
International Marketing Manager
Latin America and the Caribbean
