Introduction
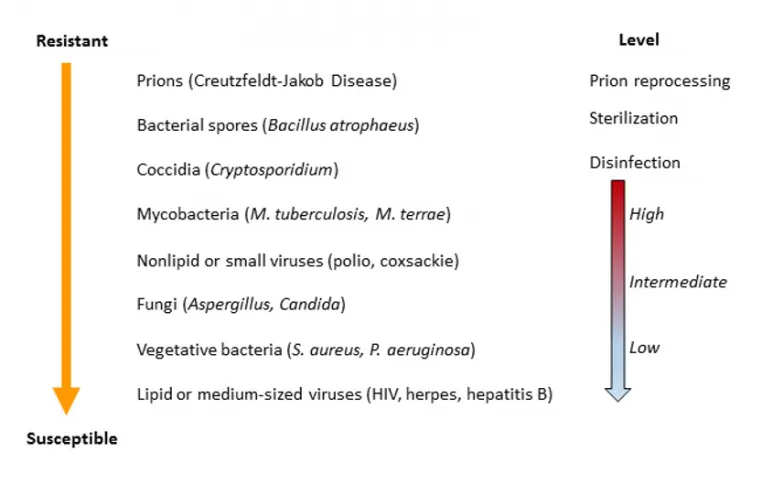
Illustration 1 and Table demonstrates the decreasing order of resistance of microorganisms to disinfection and sterilization and the level of disinfection or sterilization and the average thermal death times of vegetative stages of microorganisms. 1,2
To date, there is no information about the survival of the Covid19 virus in the environment. However, the epidemic suggests that it is quite stable, and there are studies with related viruses from the same family, e.g., SARS. The genome sequence of the virus suggests that it is about 80% similar to SARS Corona virus of 2003.3
| Microbial Type | Temperature | Time [min] |
| Non-Spore-forming bacteria | 58°C | 28 |
| Non-Spore-forming bacteria | 61°C | 18 |
| Vegetative stage of spore-forming bacteria | 58°C | 19 |
| Fungal spores | 76°C | 22 |
| Yeasts | 59°C | 19 |
| Viruses | ||
| --- Non-enveloped | 57°C | 29 |
| --- Enveloped | 54°C | 22 |
| Protozoan trophozoites | 46°C | 16 |
| Protozoan cysts | 60°C | 6 |
| Worm eggs | 54°C | 3 |
| Worm larvae | 60°C | 10 |
Survival at room temperature
Survival studies with SARS and other surrogate viruses were carried out, presenting the following of viruses survival results 20°C; body fluids, sputum, serum, and surface – 4 days; respiratory secretions – 4-5 days3; drying on plastic material – 6-9 days; drying on porous material (papers) – 4 days4. At 4°C survival is longer, up to 4 weeks4.
Survival at elevated temperature
Survival time of viruses at 56°C, 67°C and 75°C is 90 min, 60 min and 30 min, respectively. Based on the studies4, for every 10 degrees centigrade rise above 56, kill time for this virus for complete inactivation (at least 6 log), gets shorter significantly. One can deduce that at 100°C, kill time will be around 5-7 min.
Tuttnauer recommended process
Based on the above, has been recommended5 that the pre-heating stage to inactivate SARS- CoV-2 on all kinds of surfaces, as a pre-cycle prior to a regular sterilization cycle – to be carried out at 100°C for 7 min5.
A pre-heating stage was added to the sterilization cycle in order to minimize the risk for airborne contaminations, such as SARS-CoV-2, that may occur, especially in close proximity to the autoclave, during the first stage of certain sterilization cycles in autoclaves. The pre-heating stage parameters are 15 min at a minimum temperature of 103°C.
No air exhaustion into the environment occurs during this pre-heating stage. Once this pre-heating stage is completed, the standard and regulated sterilization cycle begins.
The purpose of the current tests was to demonstrate that this additional pre-heating stage can successfully disinfect vegetative bacteria, simulating a virus contamination.
Material & Methods
Bacterial strains and culture preparation - the biological experiment
The Bacillus subtilis strain harboring a green fluorescent protein (super-folder GFP, sfGFP) was obtained from the bacillus genetic stock center (http://www.bgsc.org). The B. subtilis strain (Gene Descriptor, TrpC2, amyE:(Pveg(+1/+8)_R0_sfGFP_SsrA(SVN)_spec), BGSCID 1A1270) was inoculated into 100 ml LB broth containing 100 μg/ml Spectinomycin and incubated overnight at 37°C with shaking. A sample of 1.5 ml bacterial culture were taken into an Eppendorf tube and centrifuged at 13000 rpm for 3 min. The supernatant was removed, and the cell were re-suspended in 500 μl fresh LB. The B. subtilis cell suspension was transferred into a 20 ml glass scintillation vial, placed in a pouch with a metallic load, and immediately inserted to the autoclave. An additional sample, with the same bacterial concentration was left untreated as a control.
Cell plating and counting
The vegetative B. subtilis culture vas successively diluted in fresh LB media. A 20 μl sample of stock cell culture was diluted into 1980μl sterile media forming a ×100 dilution. Successive dilution was ×4000, ×160,000 and ×6,400,000. To estimate cell population, 50 μl sample from ×160,000 dilution and 100 μl ×6,400,000 were each spread on a solid LB agar plate containing 100 μg/ml spectinomycin.
Plates were incubated at 37°C overnight and colonies were counted subsequently. This procedure was repeated 3 times for each of the tested cycles, i.e. the
pre-heating window (15 min at a minimum temperature of 103°C) with no air exhausting to the environment. Samples after autoclave treatment (the pre-heating window) were plated without dilution.
Once removed from the autoclave, 100 μl from the treated culture was plated on a solid LB agar plate containing 100 μg/ml spectinomycin. Plates were incubated at 37°C overnight and colonies were counted subsequently. This procedure was repeated 3 times for each of the tested cycles, each cycle containing 3 bacterial samples.
Culturing samples after pre-heating stage treatment
Bacterial cultures after treatment, once samples for cell counting were removed, were transferred into tube containing 6 ml fresh sterile LB liquid media containing 100 μg/ml spectinomycin. The optical density was measured at 600 nm (OD600) before incubation. Tubes were incubated at 37°C with agitation for 24 hours. After incubation, the OD600 of the samples was measured again in order to asses bacterial growth.
Validation of the complete sterilization cycle
There is no need to validate the complete conventional steam sterilization cycle since it is a proven and completely efficient well-known method.
Results
Initial cell population are demonstrated in table 2. The cell count for each of the initial samples before the cycle treatment was on average 7.6×10 8 cells for each scintillation vial.
| Sample No | CFU, ×160,000 dilution, 50 μl plating | CFU, ×6.4E6 dilution, 100 μl plating | Average cell count |
| Sample 1 | 255 colonies | 12 colonies | 7.92×108 cells |
| Sample 2 | 272 colonies | 14 colonies | 8.8×108 cells |
| Sample 3 | 212 colonies | 9 colonies | 6.3×108 cells |
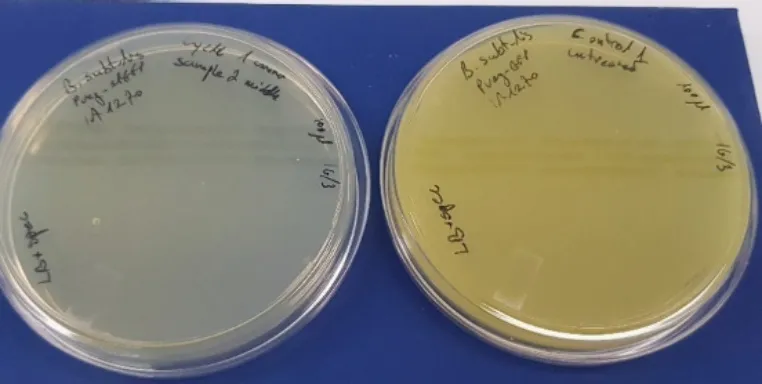
Pre-Heating Window Plating
Plating the equivalent samples, that were treated in the autoclave with pre-heating window (15 min at minimum temperature of 103°C) with no air exhausting to the environment, are demonstrated in table 3. As is can be seen, the treated samples did not exhibit bacterial growth when plated on a solid LB agar plate without exception.
| Cycle No. | Sample 1 (100 μl) | Sample 2 (100 μl) | Sample 3 (100 μl) | Control (untreated) (100 μl) |
| 1 | No growth | No growth | No growth | Growth |
| 2 | No growth | No growth | No growth | Growth |
| 3 | No growth | No growth | No growth | Growth |

Transferring the remainder of the heat-treated bacterial sample to fresh LB media did not result in bacterial growth. The remaining cell suspension (after plating) was cultured in liquid media and incubated for 48 hours. The optical density OD600 was routinely checked in all samples during the incubation time. No change in the OD600 was observed in all tested samples, indicating a lack of bacterial growth.
Summary & Conclusions
The purpose of the current tests was to demonstrate that the added pre-heating stage can successfully disinfect vegetative bacteria simulating a virus contamination.
The pre-heating stage was added to the sterilization cycle in order to minimize the risk for airborne contaminations, such as SARS-CoV-2, that may occur, especially in close proximity to the autoclave, during the first stage of certain sterilization cycles in autoclaves.
The pre-heating stage (15 min at a minimum temperature of 103°C) was tested using a biological load simulating viral contamination. The tested load, live vegetative bacteria at a concentration higher than 1×10 6 cells, was inactivated during the pre-heating stage.
No evidence of bacterial growth was recorded in all tested cycles.
About Tuttnauer
Tuttnauer provides end to end sterile processing solutions for dental and ophthalmic clinics, including; advanced autoclave sterilizers, washer-disinfectors, indicators, and sterile processing products. The new Class B autoclave with a virus protective shield is a highly advanced autoclave explicitly developed for dental, ophthalmic and medical practices by providing an extra layer of protection. The Autoclave goes above and beyond standards by meeting all sterilization needs and creating cyclic parameters that accommodate with the most challenging loads, ensuring load sterility, efficient drying, helping dentists achieve today's challenging workloads and provide superior patient care, without risking cross patient contamination.
References
- Decreasing order of resistance of microorganisms to disinfection and sterilization and the level of disinfection or sterilization, Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare. CDC guideline for disinfection and sterilization in healthcare facilities, 2008
- Average Thermal Death Times of Vegetative Stages of Microorganisms. Talaro, K.P.; Chess, B.; "Foundations in Microbiology", Physical and Chemical Agents for Microbial Control, 7th Ed., Chap. 11, The McGraw-Hill Companies, Inc., 2008
- Geler, C.; Varbanov, M.; Duval, RE.; “Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies”, Viruses. 2012 Nov 12;4(11):3044-68. doi: 10.3390/v4113044.
- Casanova, LM; Jeon, S.; Rutala, WA; Weber, DJ; Sobsey, MD; “Effects of air temperature and relative humidity on coronavirus survival on surfaces”, Appl Environ Microbiol. 2010 May;76(9):2712-7. doi: 10.1128/AEM.02291-09. Epub 2010 Mar 12.
- Heat inactivation of SARS-CoV-2. Eitan Israeli, heat process inactivation recommendation, Israeli Biohazard Ltd., recommendation letter, 2020.