The following article examines key features to consider when purchasing a laboratory autoclave. By the end of this article you will be able to answer the following questions:
- How to reduce cooling times?
- Waste sterilization and the biohazard filter
- Why are PT 100 sensors necessary for sterilizing liquids?
- When is Vacuum-Assisted Air Removal used?
- What are the benefits of advanced reporting software
Liquid sterilization with super-fast cooling
Fast cooling reduces cooling time by up to 75% compared to cooling under ambient conditions.
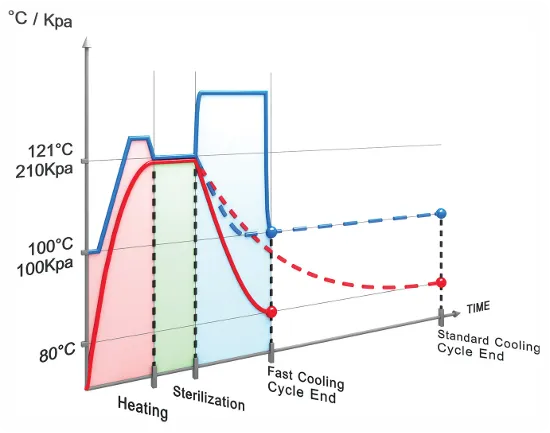
How does it work?
Following the sterilization phase, the chamber pressure is increased by forcing compressed air through a filter into the chamber. Increasing the chamber pressure prevents boil-over of liquids, spills, or cracks in containers that may occur under low-pressure conditions. During this phase, cold water circulates through coiled pipes that surround the chamber. Due to the circulation of cold water and the pressurized air, the liquid load is cooled quickly and safely, saving valuable time and protecting the sterilized media from overboiling. Super-fast cooling is combined with fast cooling by using a radial ventilator. By circulating the air in the chamber, it transfers the heat from the load to the relatively cooler chamber walls, cooling the sterilized load by as much as 90% more rapidly.

Fo autoclave cycle for heat-sensitive liquid media
Tuttnauer’s laboratory autoclaves perform Fo cycles (pronounced F sub-zero). This feature reduces the exposure time of the load to high temperatures. It is especially relevant when sterilizing delicate heat-sensitive liquid media. By minimizing the time liquid media are exposed to high temperatures, the integrity of the load is maintained, as well as reduced overall cycle time. The theory behind F0 is that microorganisms and bacteria can already be destroyed before hitting the sterilization start point (e.g., 115°C.), and therefore, actual sterilization can begin before the start point is reached. The F0 Cycle is primarily designed to give you “credit” for the sterilization that happens while your load is coming up to temperature.
How does Fo work?
There are two methods to apply the F0 cycle:
Starting at 105°C, the heat-up time is calculated to contribute to the total sterilization time. The first option calculates the total F0 value but does not reduce the total cycle time accordingly. It merely tracks and reports the F0 value at cycle end. The second option will adjust the sterilization hold time according to the F0 value, which depends on different variables, including load size. This option reduces sterilization hold time according to the F0 value. If a standard sterilization holding time is set to 15 minutes, the sterilization hold time may be reduced based on the F0 calculation by minutes. Resulting in saved time and avoiding overcooking the liquid while still ensuring appropriate sterilization.
Waste sterilization with Biohazard filter
Does your laboratory work with Covid-19 test samples or biohazardous media? Air discharged from the autoclave may contain infectious substances, potentially contaminated by toxins, viruses such as the novel Coronavirus, or medical waste. Biohazardous media put at risk both the staff handling the media, as well as the lab environment. It is essential to have an appropriate sterilization solution that protects the lab environment from this potentially harmful air affluent. Therefore it is highly recommended to equip the autoclave with an advanced air filter.

How does it work?
During pre-sterilization air removal and heating stage, the chamber air is passed through a microbiological filter that filters harmful substances. Air is filtered by a High-Efficiency Particulate Air filter, known as a HEPA filter. HEPA filters are used to block the passage of microorganisms found in the autoclave effluent. The destruction of pathogens caught inside the filter not only eliminates them and maintains the sterile conditions of the surrounding lab, but it also allows for safe, sterile conditions for the following autoclave cycle. Tuttnauer’s horizontal and vertical autoclaves are optionally equipped with a HEPA air filter that blocks microorganisms from exiting the chamber during the air removal phase. This protective measure prevents the contaminated air from escaping into the atmosphere and, instead, catches the harmful microorganism inside exhausting decontaminated air, a feature that cannot be overlooked in post-COVID-19 times.
PT 100 Sensors
There is a significant gap between the load and the chamber heating time when sterilizing liquids, the former being significantly slower than the latter. The temperature in the chamber reaches sterilization conditions long before the liquid. For example, the chamber temperature may reach 121°C, initiating the sterilization phase; however, the liquid has not yet reached sterilization temperature. Therefore the process will not be valid. Two PT100 sensors are placed within the liquid, ensuring the sterilization phase will only begin when the liquid reaches the sterilization temperature. In an autoclave that sterilizes solids, the sensor is typically located near the drain. Most laboratory autoclaves contain only one PT100 probe, but Tuttnauer provides a solution that goes beyond the required standard. If a flask containing a PT100 sensor explodes or cracks, the autoclave is equipped with an additional sensor for back up, providing dual safety control.

Sterilizing glassware and hollow instruments
When sterilizing glassware, hollows, and pipette tips, it is essential to remove the air before sterilization to maximize steam penetration. These loads inherently contain air inside the vessels, and this air must be removed to ensure complete sterilization of the load. Using a vacuum pump, via pre-vacuum air removal pulses, more than 99% of the air is removed even from the tallest and narrowest containers. The vacuum pump also dries the load more efficiently by keeping the pressure low and thus quickly extracting the vaporized moisture.
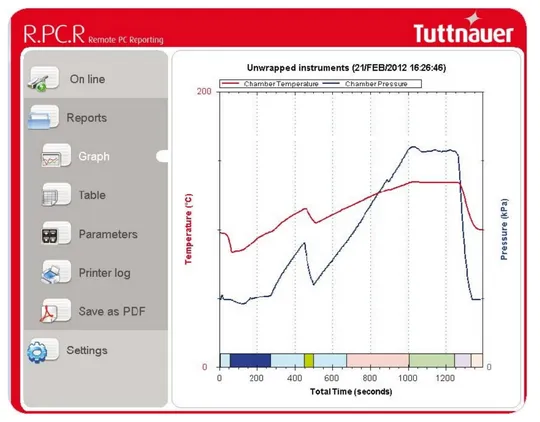
User-friendly Control System and advanced R.PC.R. software
The advantages of a state-of-the-art Control System with Multi-Color Display include:
- Fo software control
- 200 cycles storage
The Tuttnauer R.PC.R software is an essential addition to research and laboratory autoclaves. It performs automatic cycle registration and can be used over the network or remotely. It is an excellent report generator that enables you to:
- Manage cycle history report files online or offline by USB
- Track the entire history of the processes run by the autoclave.
- Track-specific parameters that have been used in each cycle and stage
- Choose a reporting style including graphs, tables or printouts
- Generate PDF reports
The control system and RPCR software comply with the 21 CFR part 11 standard
Tuttnauer laboratory autoclaves are ideal for sterilizing contaminated waste, instruments, culture media, or laboratory equipment. Tuttnauer offers the ultimate autoclaves for safe and validatable sterilization with accurate documentation in a wide range of models, sizes, and door configurations.

About Tuttnauer: Tuttnauer provides end to end sterile processing solutions for laboratories, research institutes, hospitals, medical, dental and ophthalmic clinics, including; advanced autoclave, washer-disinfectors, indicators, and sterile processing products. The safe and reliable biocontainment systems are designed to deal with the environmental threat posed by pathogens and other contaminated materials emitted from laboratories. Tuttnauer takes the most extreme measures in fighting bacteria and viruses, including the Coronavirus (aka SARS-CoV-2), by providing innovative decontamination solutions that go above and beyond the required standards.
For further information and to inquire about purchasing the advanced autoclave for your laboratory, contact a Tuttnauer representative.