More information about GS Hospital Autoclaves
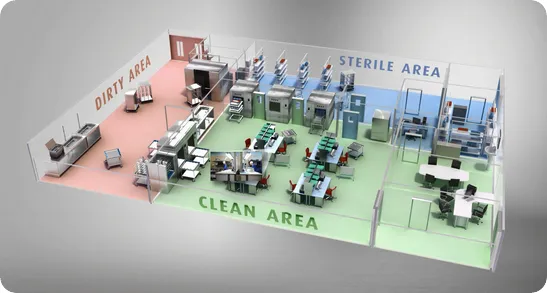
The standard and best practice central sterile services department is divided to three different sections. Tools used to treat patients on the wards, emergency rooms and in the operating theater go to a dirty area, they are processed and cleaned before being taken to the clean area. They are then sterilized and put into the sterile area.
In the dirty area everything is delivered to be processed. There is the initial pre-sterilization and cleaning process, for example, an ultrasonic cleaner may be used to clean dried blood off of tools.
Washers will then be used, similar to a washer you have in your kitchen. It will use many harsh chemical detergents to clean the tools before the tools are moved to the clean area.
The clean area will generally have tables for people to package the tools that came out of the washer. They can wrap them if they know that the tools aren’t going to be used immediately and they need to be stored on a shelf for a few days, weeks or months.
If the tools are to be used immediately, for example in another surgery, they are left unwrapped. Once all the items are sorted and either wrapped or left exposed the are put into the autoclave for sterilization.
In the gold standard CSSD, the autoclave will have a door leading to the clean area and a door on the other side leading to the sterile area, so the items can be put in on one side, the sterilization cycle can be run and the tools can be taken out sterile into a sterile environment.
The prevalence depends on the type on environment and the conditions of the medical setting. For example, in an infectious disease research lab having a pass through autoclave is mandatory to prevent the spread of disease leaving the research area.
In CSSD’s they are quite prevalent, especially in Western and developed economies, where there are resources and a strong desire to combat infectious pathogens. We have found an increasing demand for pass through autoclaves such as our GS line in both Europe and North America.
However, while it is good practice to separate the dirty area from the clean area and the clean room from the sterile room, it is not mandatory. In medical settings where there isn’t space or resources for three separate areas you may find single door autoclaves used.
Often clients will contact us during the preparation of structural designs, we can advise on the size an opening must be for the autoclaves that the center would like to use. Then we can push the autoclave into the prepared gap and close it up. Sometimes it is sealed, sometimes it doesn't have to be sealed, depending on the setting.
We can retrofit an autoclave into a space, if a hole needs to be made then that can be achieved, but most of the time hospitals tell us in advance.

When we refer to hospital grade performance of our autoclaves we are displaying that we produce the highest level of the autoclave according to national and international standards for example in Europe we comply with the European standard EN285.
In an autoclave there are many different cycles. There is a glass cycle, plastic cycle, wrapped and unwrapped cycle. You cannot, for example, run plastic in a glass cycle.
The equipment in the glass and plastics cycles are usually those left unwrapped, neither is better than the other, they simply serve different functions. A wrapped cycle, is where tools have been wrapped up and will remain wrapped until use.
That use could be days or weeks from now, but we will need those tools and we can’t wait until then to sterilize them, as they might otherwise spread disease. We also don’t want to sterilize them twice, once immediately after use and once before they are used again, as that is a waste of resources.
So a wrapped cycle allows you to sterilize the tools and the small wrapped environment that they are in, allowing us to know that they will remain sterile until they are needed.
On the other hand, an unwrapped cycle is for tools that are going to be used immediately, for example, in smaller medical environments like your dentist’s, they aren’t going to have hundreds of knives.
Instead maybe a couple of sets, which they may need to use repeatedly on different customers throughout the day. They can sterilize a set while taking care of a patient and use the newly sterilized set with the following patient.
The GS line has been designed for customers that don’t need the typical horizontal autoclave with square chamber design, but still need hospital grade performance. The horizontal autoclave is a complicated autoclave to manufacture, which makes it expensive. You have to produce the chamber, and weld a structure around the chamber, which we call the jacket and so on.
The GS we have designed an autoclave chamber without any need to build a jacket around it, making it more cost effective. It's much more simple to build rather than typical horizontal autoclave with square chamber design and it is also easier to maintain.
This design is also narrower making it more able to be fit into tight spaces that the horizontal square chamber models have not been able to reach.
The doors have been made much more user friendly on the GS than the horizontal square chamber autoclave where we have additional features for automatic distance reduction and moving it up and down.

The on board control system will count the cycle number with every cycle, and store the information of the last 200 cycles in its memory. The temperature and pressure measurements over the duration of the cycle can be checked at a later date.
We have an option to activate bar code tracking. If you use this system, you will know which tools you're putting inside. This helps you keep track of the often expensive tools, so for example, if a piece of equipment such as a tube and camera used for colonoscopy should receive regular maintenance after 100 cycles you can easily keep track of how many times it has been used and take it out of circulation at the right time, rather than guessing.
The GS provides print outs on both sides of the autoclave to help each side keep track of the cycles being run and also sends out real time data on each cycle.
Two files are sent to a networked PC, the first is a txt file, which is exactly the same as the printout. Another file is the cycle number and associated cycle data, called the CYC. The CYC file, can be uploaded to a PC with ‘Remote PC Reporting’ (RPCR) software installed.
In the RPCR you can upload the cycle and you can create a pdf file with the cycle, with the parameters, with the users, with everything that was inside the data.
There are many automatic safety mechanisms in our autoclaves, which all come as standard. We have a safety valve that makes sure the steam pressure doesn’t get too high and compromise the autoclave, so that the chamber doesn’t over pressurize.
Our doors have micro sutures, which if moved from the on to off position because the door has moved or something similar, it will stop the cycle automatically. This is something that has never happened, but we have the safety feature in place just in case.
If water remains in the chamber once a cycle has ended, the water will be really hot inside, which could scald the operator if the door was opened and water spilled out. To combat this, we have a sensor that seals the chamber if there is left over water. If there is water and it wasn't drained to the sewage, you won't be able to open the door as well.
The technicians can also use our diagnostics tests to check each component of the system independently for peace of mind.
I would say that two door autoclaves are the standard to go for at the moment, and that we will sell more in the future, especially to Western markets.
The GS system can also be used flexibly, it can be configured to be a one door system, and then when the hospital upgrades it can be reconfigured as a two door system.
We are always thinking about for the future and innovating to meet our customers’ needs.
