Before the Coronavirus pandemic, many dental practices had a level of confidence in their sterilization routine. Today, dental practices need to reassure their patients and their staff, that their instrument reprocessing procedure follows a gold standard.
In this paper, we will outline the key testing tools available for cleaning. In some cases, IFUs (Instructions For Use) require to perform this more frequently than mandated by regulatory organizations.
This is because frequency is the key to optimal safety control.
Instrument Cleaning Efficacy
Instrument thermal high disinfector washers and ultrasonic cleaners
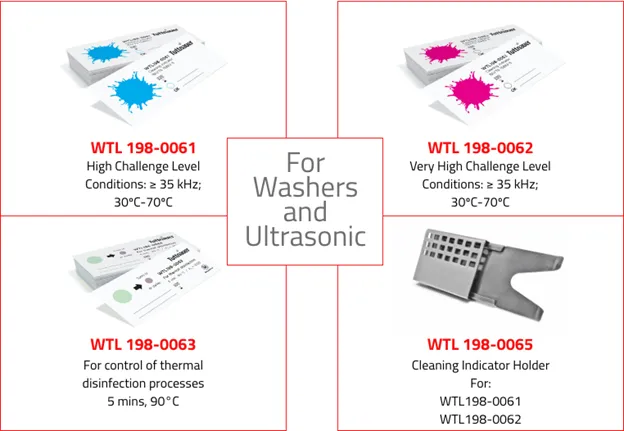
The IFU may recommend testing for each load. Cleaning efficacy tests consist of a strip with a substitute soil containing proteins, carbohydrates, fatty acids and non-toxic dyes. The soil mimics that found on contaminated instruments. The cleaning indicator is included along with the load to be cleaned and examined after completion of the cycle. If all ‘soil’ has been removed, cleaning was effective, alternatively, ineffective if any remains.

Tuttnauer’s product lines include cleaning indicators, which are designed for routine control of cleaning processes for monitoring the effectiveness of automatic high thermal disinfector washers and ultrasonic cleaners. These indicators will allow a quick visual evaluation and identify the potential source of error.
Protein Residue Test
Washer-disinfectors
In addition to these and the routine tests discussed in this article, filters in instrument washer-disinfectors should be checked, removed, and cleaned regularly as well as checking the condition of the door seal as a safety check.
Additional testing for instruments
Instrument washer-disinfectors, ultrasonic cleaners and manual cleaning
As its name suggests, the protein residue test is performed to determine whether there is any residual protein on cleaned instruments. For a protein residue test, a swab is taken from a randomly selected instrument that has been cleaned. This swab is then immersed in a tube containing a custom dye after which the liquid is swirled for 5 seconds. If the color changes to a shade of blue, this indicates that proteins are present. The darker the shade of blue the more protein is present.
Washing Device Cleaning Inspection
Instrument washer-disinfectors, ultrasonic cleaners and manual cleaning
After cleaning, all instruments must be inspected to check that they are thoroughly clean and free of debris. This inspection should occur under illumination. Magnification may be required. If any debris is found, the device must be cleaned again. Manual cleaning may not be permitted in your location unless it is the only option, or for isolated spots. It is also discouraged.

Ultrasonic Activity Test
Ultrasonic cleaners
In order for ultrasonic cleaning to be effective, the ultrasonic cleaner must have an appropriate and even level of transducer activity, which results in appropriate cavitation and implosion within the solution, in all areas of the chamber. Without this, instruments will retain soil. Options include the traditional foil test and recently introduced dye tests.
Dye test
The dye test is easier to perform, simple, and provides a clear visual result that is not open to interpretation. It consists of glass vials that are placed in an ultrasonic cleaner rack in a configuration determined by the area, in accordance with the IFU. The vials contain small beads and a blue reactive dye at the start of the test prior to running the ultrasonic cleaner. During cleaning, the beads become agitated causing the dye to change color. If the ultrasonic cleaner is functioning properly, the dye will have turned yellow. If ultrasonic activity is reduced, the dye will change to a shade of green.

Foil test
The foil test is performed using strips of regular aluminum foil configured as recommended. This can consist of nine pre-measured strips adhering to adhesive tape in a 3 x 3 grid. Fresh ultrasonic cleaning solution should be placed in the chamber, measured to the correct level and at the recommended temperature, as well as degassed. While foil testing is used in some cases – it is not the recommended testing for the ultrasonic cleaners. The dye test using vials is the recommended testing for ultrasonic cleaners, as a more accurate alternative, as described above.
Summary
Additional tests are required for validation and periodic testing, but this is beyond the scope of this article. Testing by in-office personnel (including its frequency) and periodic servicing and validation should be performed in accordance with the recommendations and regulations in your area, and the manufacturers’ IFU for the reprocessing devices you are using. Thorough cleaning is critical, and that makes testing critical too.
Visit Tuttnauer’s website product line to learn more about the most effective reprocessing sterilization methods and the necessary products to achieve optimal safety control.
Resources
- Association for the Advancement of Medical Instrumentation. ANSI/AAMI ST79: 2017. Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
- Dubai Health Authority. Health Regulation Department. Guidelines on Dental Infection Prevention and Safety.
- CDC. Guidelines for infection control in dental healthcare settings – 2003.
- Health Technical Memorandum 01-05: Decontamination in primary care dental practices. (2013)
