In an effort to do the correct thing, dental offices are more often using chemical indicators, thinking that one is needed for the outside of the package and one for the inside of the package. What they don’t realize is that there are very different types of chemical indicators. Instead of using the appropriate chemical indicator for the outside of the package and the correct one for the inside of the instrument package, dental practices are often using the external chemical indicator for both. This is a mistake often being made despite good intentions. In this article, we will review the proper way to use chemical indicators when sterilizing reusable dental instruments.
Instrument processing is an essential part of daily operations in every dental office. Reusable instruments undergo various sterilization processes to remove infectious material from the previous patient to prevent cross-infection to the next patient or any dental team member.
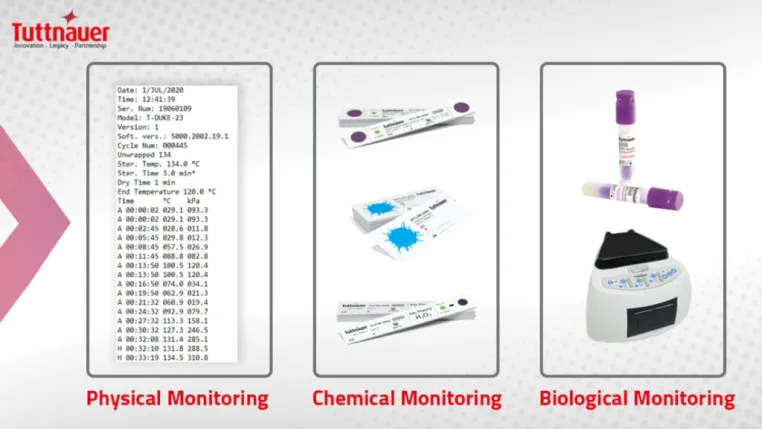
To verify that the items are clean, sterile, and, more importantly, safe to use on the next patient, the Centers for Disease Control and Prevention (CDC) recommends using a combination of biological (BI), chemical (CI), and physical/mechanical indicators (MI) to monitor the effectiveness of the sterilization process.
The use of Indicators in monitoring sterilization
The current gold standard for monitoring is biological indicators or spore tests, which monitor the functioning of autoclaves by directly evaluating whether any highly resistant microorganisms are left behind during the process. A negative or Inactivated BI indicates that all pathogens in the load have been eliminated, therefore confirming sterilization. However, the performance of biological monitoring is only required on a weekly basis as it takes time to produce results.

Chemical indicators allow us to verify the proper sterilization procedures of instruments in between biological monitoring. Mechanical monitoring can detect equipment malfunctions by ensuring that any machinery involved, such as the autoclave machine, is operating optimally, at the right temperature, time, and for the correct duration.
While they do not confirm sterilization, chemical and mechanical indicators expose procedural errors and equipment malfunctions during reprocessing. Therefore, they must be carried out for every sterilizer load to detect discrepancies, alongside using a biological indicator once every week to ensure successful disinfection.
Understanding different types of chemical indicators
Chemical indicators are an affordable, convenient, and effective way to determine if an item has been through a sterilization cycle.
There are six classes of CIs, and the CDC recommends using at least two: an external (Type 1) and an internal (Type 3, 4, 5, and 6) marker, to be placed on the inside and outside of every instrument package. It is important to note that this classification has no hierarchical significance. Type 6, for instance, is not better than Type 1; they simply measure different metrics. Therefore, deciding which indicator to use for the task at hand merely depends on the required measurement parameters.
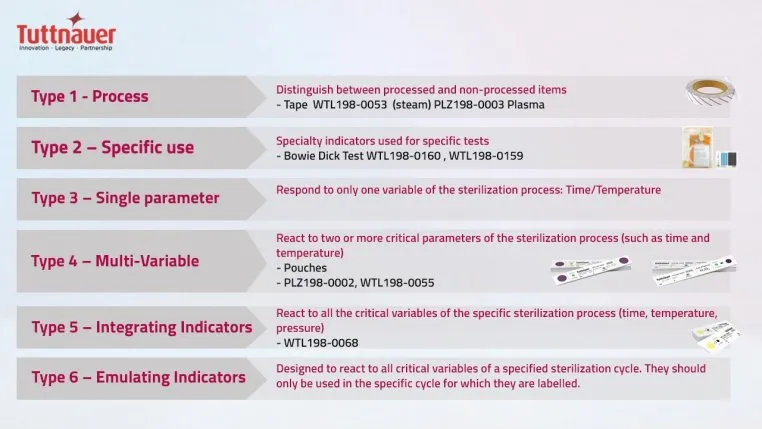
Indicators on the outside (Type 1) show whether an article was heat exposed, which means it has undergone the disinfection process and makes it easier to distinguish processed from unprocessed items.
Internal indicators demonstrate the achievement of sterilization parameters like time, temperature, and pressure. By assessing these variables, chemical indicators show the completion of the sterilization process or any failings in the process.
Type 2 CIs are specific; they assess the efficacy of air removal in a prevacuum sterilizer. Type 3 indicators identify exposure in specific places by measuring only one variable, either temperature or time. Type 4 is a multivariable marker since it can respond to more than one indicated parameter. Types 5, such as the Tuttnauer Chemical Indicator, and 6 can react to all critical parameters and are usually the go-to choice for ensuring sterilization success.
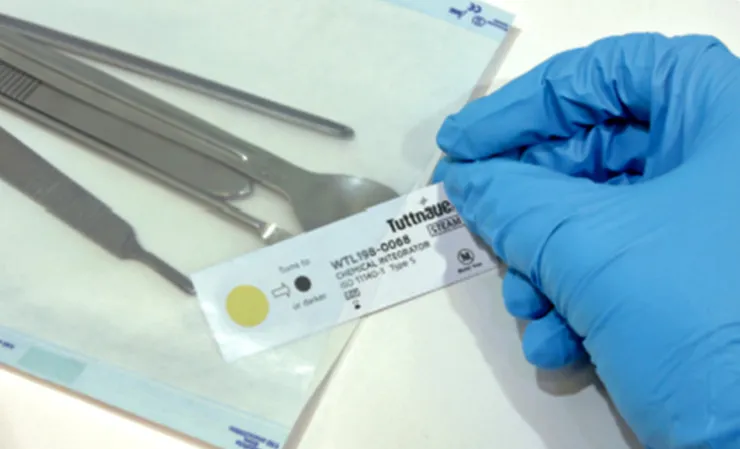
Type 5 internal indicators mimic the response of biological indicators, and some experts consider them to exceed their performance requirements. They clearly demonstrate the effectiveness of the sterilization process by changing color when the sterilizing agent (e.g., steam) successfully penetrates the material and has satisfied all critical parameters.
Best practices for sterilization monitoring
Decisions on when to use which type of chemical indicator depend on the sterilization packaging. When using pouches, the placement of a type 4 internal CI, which is clearly labeled for traceability, is essential inside the package. When utilizing cassettes, each cassette should have a wrapped and labeled type 5 indicator and the case secured with a type 1 tape. Note, although type 5 indicators mimic the response of a biological indicator, they test only the specific package they were placed into, as appose to a BI which test the entire autoclave chamber.
As a rule of thumb, chemical indicators should be used inside and outside each package to show that it has been through a sterilization cycle. Furthermore, color change or CI alone is insufficient to demonstrate sterilization. Examining all physical, biological, and chemical parameters is required to ensure the sterility of the items.
Chemical indicators are essential to ensure the complete and successful sterilization of dental instruments. In combination with physical monitoring and regular spore testing, indicators assist practices in maintaining high standards for reprocessing, thereby reducing the risk of infection.
However, monitoring should not stop at the autoclave. We should also use indicators in other equipment, such as Tiva Washers. It is essential to ensure that they are checked for efficiency too. Products such as Tuttnauer cleaning and disinfection indicators can make this process much more straightforward and effective.
The correct processing of instruments is an essential pillar of infection control. By implementing and following strict standard operating procedures, favorable results are achievable with a low risk of cross-contamination.
When employed as part of a comprehensive quality and infection control program, indicators detect problems or errors that could result in non-sterile equipment. Relevant staff members should be well trained on the procedures and understand the rationale for carrying out these tasks that significantly impact patient safety.
Although there have been significant changes in the dental industry, particularly due to COVID-19, sterilization monitoring remains a constant and vital quality assurance process. It provides peace of mind that staff and patient safety are safeguarded and upholds a high standard of care for the practice.

