We use heat to perform sterilization and our carrier is moisture in an exact value. In order to achieve an effective sterilization process, we should have control of each one of the three parameters combined, in order to produce saturated steam. A controlled process will produce the exact percentage of saturation that will carry the heat onto the microbes.
It is also important to maintain this control over time and over the entire sterilization volume, as we learn from the ideal Gas equation:

The cycle requirements for every load type can, however, vary significantly. This article introduces five different sterilization cycles commonly used in a cGMP pharmaceutical setting. The three sterilization cycles are: Gravity, Pre-vacuum and Air Steam Mixture Cycle, and the two test cycles: Bowie and Dick and Leak Test.
The Gravity Cycle is the simplest cycle; ideal for sterilizing liquids, media, glassware & plastic, culture plates and unwrapped instruments.
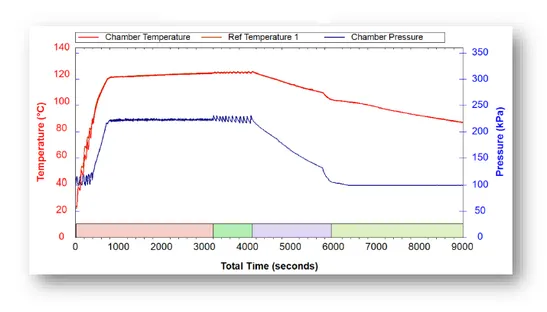
The cycle begins with a short heating phase as steam is introduced into the chamber (Up to around 1000 seconds in the above graph). As steam fills the chamber, the air is forced out through a drain vent. By pushing the air out, the steam directly contacts the load and begins to sterilize it. Sterilization occurs when a pressure of 208 kPa and a temperature of 121℃ are reached. The sterilization phase (Seconds 3000-4000) is approximately 1000 seconds long. Afterwards, the system and load are cooled down at a very slow pace that can take up to 10 hours. The slow cool-down prevents liquids from boiling over and spilling. There is an option to add a fast cooling system that decreases cycle time by up to 80%.
Used to Sterilize:
Disadvantages:
Advantages:
When dealing with hollow loads, solids, wrapped packages or porous loads, effective sterilization means removing as much air as possible in the first stage, prior to sterilization. For these loads the Pre-Vacuum Cycle was designed.
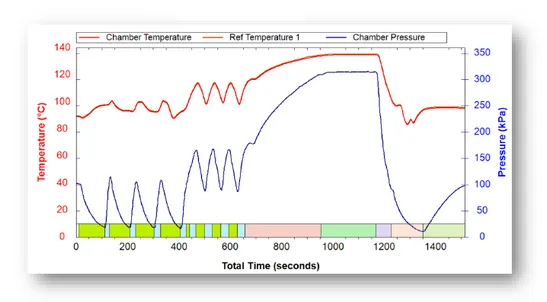
The Pre-vacuum cycle actively removes air from the chamber prior to the sterilization phase, using a vacuum pump. It is used for sterilizing hollow loads, porous loads and wrapped packages. The cycle begins with 4 pulses of vacuum, gradually removing air from the chamber and inserting steam into it. Eventually more than 99% of the air is removed and we are left with 0.016% of initial air (after 400 seconds in the above graph). After that we gradually heat the chamber, creating a homogenous temperature throughout the volume. Sterilization then occurs (Seconds 1000 to 1200).
After sterilization, the chamber and load are cooled down. When the pressure drops, all residual moisture boils and is transformed into a gaseous state. When pressure is released from the chamber, the moisture exits as well, leaving our product sterilized and dry.
Used to Sterilize:
Advantages:
Disadvantages:
The Air Steam Mixture Cycle (ASM) is used to sterilize liquids that are sealed in a package. These include contact lenses, glass bottles, ampoules and infusion bags. It is designed to sterilize large quantities; for example, 25,000 ampoules, 20,000 infusion bags or 30,000 syringes. Packaging integrity is maintained and there is no deformation of elastic packages.
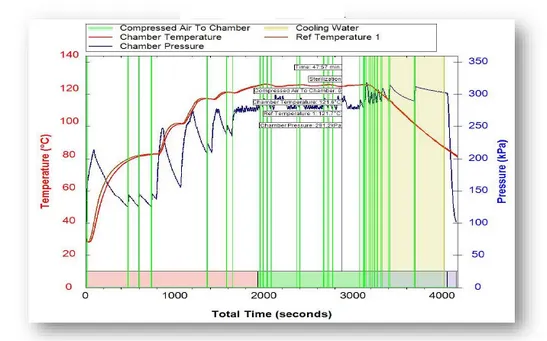
In ASM cycle the sterilization media is a mixture of steam and air; i.e., air removal is not an issue as the loads are sealed (Ampule for example). Throughout the cycle overpressure is maintained; the pressure external to the load is higher than internal pressure. Temperature is gradually increased to achieve a homogenic temperature increment (Seconds 0-2000 in the above graph). This state of overpressure is maintained throughout the sterilization process. After the sterilization cycle ends, the temperature is slowly reduced by water that is introduced into the jacket. We start the cooling using air, while maintaining constant high pressure and thus avoiding boiling (low pressure means lower boiling temperature). Once the temperature is below 80℃, the air is released and the load is ready for usage. The end result is a sterile product that is dry and ready for packaging.
Loads:
Advantages:
The Bowie & Dick Test (B&D) indicates proper air removal from the chamber of a pre-vacuum autoclave. It ensures that all sterilization parameters and processes are in order. The B&D test is a chemical indicator inserted in a test pack. The test pack is placed into an empty chamber, and a pre-vacuum cycle is then activated. If the test fails, it indicates that the autoclave has leak problems.

As we can see in the picture above:
The Leak test or Vacuum test is designed to examine air-tight integrity and the presence of leaks in an autoclave chamber and pipes.
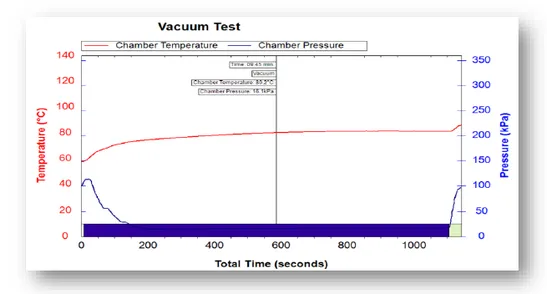
The chamber is brought to vacuum conditions of 7 kPa (after approximately 200 seconds in the above graph), and all valves and motors are closed for 5 minutes, enabling pressure stabilization. A typical Vacuum Leak Test Cycle will consist of three alternating vacuum and pressure pulses, followed by a 15-minute dwell period at deep vacuum. The permitted pressure change over the following 10 minutes is 1.3 kPa or less.
The cycles described in this article are the basics of sterilization processes in a pharmaceutical autoclave. Different loads require different sterilization cycles and in order to support this we offer the following options as well:
These are only some of the processes available with Tuttnauer's autoclaves, and there is a fit for every possible load.
About Tuttnauer - the Leading Pharmaceutical Autoclave Manufacturer
Tuttnauer is a leading manufacturer of infection control solutions for Pharmaceutical, Life Science & Healthcare industries, with over 90 years of sterilization experience and 350,000 worldwide installations. Tuttnauer designs pharmaceutical autoclaves in compliance with GMP regulations, to meet the technical challenges of pharma and biotech. Tuttnauer provides the highest quality products and full documentation. This article addresses the typical cycles used in pharmaceutical autoclaves.

