Chairside Safety Measures
One risk reduction factor is chairside disposal of sharps in a sharps container, that is puncture-proof, sturdy, and can be sealed when full, prior to disposal. The sharps container must meet requirements under national and local regulations. Chairside disposal avoids the need to transport these to the reprocessing area/room and, together with the transportation of instruments in appropriate puncture-proof containers and the use of PPE, reduces risk by removing a source of sharps injury. Risk reduction can further be achieved by using appropriate liquid-resistant attire, a fluid-resistant face mask, face shield or goggles, heavy-duty puncture-resistant and chemical-resistant gloves, closed shoes and if indicated hair covering and a water-proof apron.

Reprocessing Area
Within the reprocessing room/area itself, several technologies can be implemented that help to reduce risk. For example, a pre-soak with enzymatic technologies may be used for instruments to moisten dried-on soil or to prevent it, if time will elapse before cleaning. This decreases the likelihood of residual soil after cleaning and the possibility of needing to repeat cleaning, which might also involve manual spot cleaning that would increase risk for the operator.
Cleaning Devices - Washers or Ultrasonic
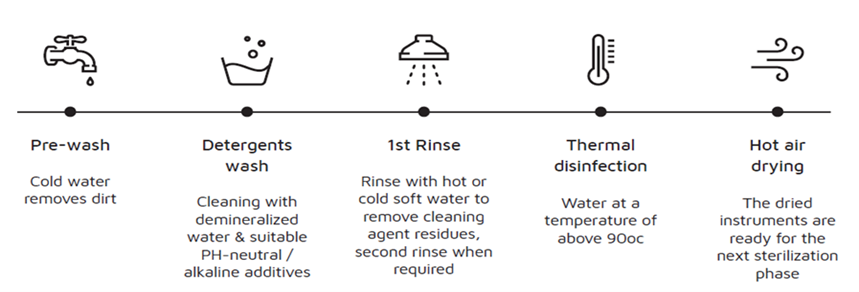
Cleaning devices such as instrument washers, instrument washer-disinfectors and ultrasonic cleaners minimize the risk of cross-contamination for all personnel. Instrument washers and washer-disinfectors fully automate the cleaning phase of reprocessing. That includes the pre-wash to remove gross soil, mechanical cleaning using a proprietary chemical formulation, a rinse to remove cleaning agent residue (and when indicated a second rinse), and hot air drying of instruments before the cycle is completed. No handling of instruments occurs during these steps, reducing risk of infection for the operator. For instrument washer-disinfectors, risk is further reduced by the thermal disinfection phase that occurs after the second rinse and prior to drying. This phase reduces the microbial load and therefore the risk of transmission should the operator encounter the instruments. Tuttnauer's Tiva 2 washer-disinfector was designed to simplify your work and ensure the safety of your patients and staff. This turnkey solution for washing, disinfecting and drying medical instruments for a complete decontamination cycle, will maximize sustainability while minimizing consumption and operation costs.

In the case of the ultrasonic cleaner, risk is further reduced compared to manual cleaning. In addition, the lid must be closed on the ultrasonic cleaner to prevent aerosolization of the ultrasonic cleaning solution. According to the CDC, better risk reduction is achieved with ultrasonic cleaners compared to manual cleaning.
Instruments always need to be rinsed and dried manually afterwards. In some locations a hot air drier may be available that can be used to dry instruments following rinsing.
h3 class="tutt-heading"style="color: rgb(51, 51, 51);">
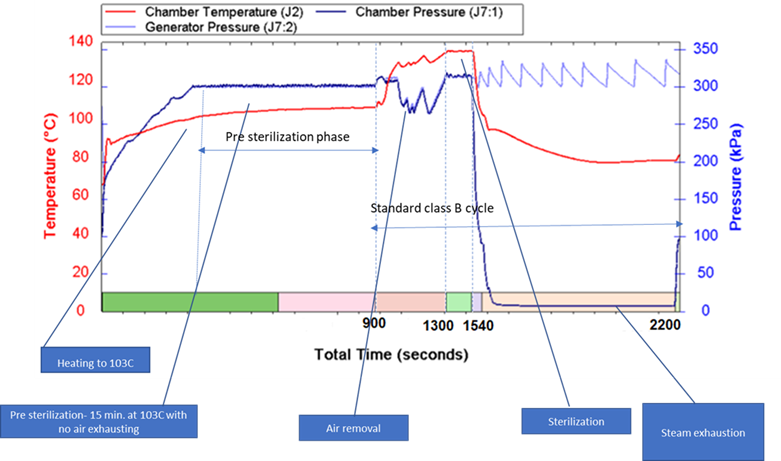
Technologies related to autoclaves (steam sterilizers) also offer potential opportunities. Air must be effectively removed from autoclave chamber and vented from the autoclave before the sterilization phase occurs. For two types of Class B autoclaves (T-Edge, Elara and HSG), an innovative pre-sterilization phase is offered as an option that decontaminates air prior to venting.
Virus Protective Shield
To date there is no evidence that vented air from autoclaves has resulted in disease transmission, however this is a new option created to reduce the microbial load. This pre-sterilization phase consists of holding the temperature in the autoclave chamber at 103 ° Celsius for 15 minutes after which venting takes place. This temperature and time combination has been shown to reduce the level of susceptible microorganisms present, which would include viruses and susceptible bacteria, and therefore reduces the amount in air vented from the autoclave prior to the sterilization phase.
Clearly, both validation and maintenance coupled with safety checks, reduces risk not only of compromised reprocessing, but also risk for personnel by preventing malfunctioning that could cause injury. Regulated medical devices have been tested and reviewed for safety based on their intended use, and this data is reviewed by regulatory authorities. National, regional and international standards (e.g., ISO) also contribute to safety by describing recommended processes, testing, operating criteria and safety criteria for devices.
In conclusion, technologies and engineering controls, work controls, following the IFU for reprocessing, validation and maintenance of devices all contribute to risk reduction during reprocessing
