In one situation, the answer was, “we don’t do any of that.” I was speechless. I dared to ask about autoclave maintenance and how often the team was cleaning and maintaining the autoclave. The answer also left me speechless. From that moment on, I assume they are doing nothing until proven otherwise.
A more recent concern while working in a new practice was the use of a mail-in spore tests. It appeared to me that the mail-in test would be run as instructed, but then it wouldn’t make it up front into the out-going mail that day. Then the mail didn’t get picked up some days, or a holiday was happening so it would be almost 5-7 days before the test reached the testing center. This means, if it failed, we had used the instruments on patients many times over by the time we got the results. This was a major concern for me. Let’s talk about why all these issues are a problem.
What happens when biological monitoring is not enforced?
Regularly monitoring the sterilizer in a dental office is integral to infection control. Biological monitoring is the gold standard for assessing and assuring proper sterilization of dental instruments.
In developing countries, inadequate sterilization of medical devices is one of the leading causes of healthcare-related infections. A study in Mexico found that 17% of sterilizers had bacterial growth due to mistakes in temperature, time, pressure, inadequate supervision, and improper maintenance. In primary and secondary care public hospitals in Nepal, 71.0% of the autoclave cycles were ineffective when tested with biological indicators, and 69.8% showed ‘reject’ results with class 5 chemical indicators.
These high proportions of inadequate sterilization increase the risk of person-to-person transmission of pathogens through reusable medical devices.
While in-office biological monitoring is more strictly controlled and enforced in the USA, when a spore test does fail, letting patients know there was a breach in the sterilization procedures and that non-sterilized instruments were used is a stressful and embarrassing situation to deal with for any office.
Recommended frequency of biological monitoring
Steam sterilization (autoclaving) is the most widely used method. It is considered the most robust and cost-effective method for medical devices, as it can kill highly resistant microorganisms.
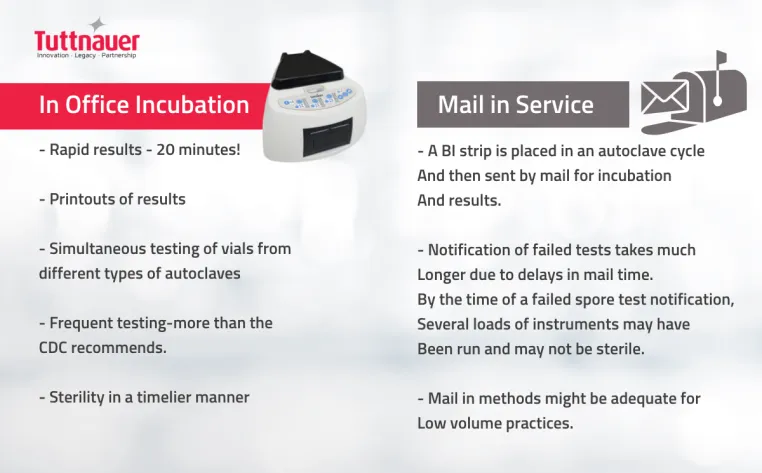
Proper functioning of the sterilization cycle should be verified at least weekly, according to the CDC and the American Dental Association. The majority of state dental boards require verification by using a biological indicator, such as a spore check system, and require spore test result records to be retained. Using an in-office spore test allows you to get the results faster and prevent any additional instruments from being run in a malfunctioning autoclave and used on patients until the failure has been corrected, rather than a mail-in system where results can take days. Because spore tests are only performed weekly, and the results are usually not obtained immediately, it is also necessary to perform mechanical and chemical monitoring.
Mechanical indicators detect procedural errors and equipment malfunctions and should be used for every sterilizer load. Checking that pressure, temperature, and exposure time have reached the levels recommended by the sterilizer manufacturer and finding discrepancies could be the first indication of a problem.
A chemical indicator should be used inside every package to verify that the sterilizing agent has penetrated the package and reached the instruments inside. Chemical indicators help to differentiate between processed and unprocessed items, eliminating the possibility of using instruments that have not been sterilized.

The importance of autoclave cleaning and maintenance
The most effective method for avoiding a positive spore test is by using adequate steam sterilization with an autoclave. The best way to ensure the autoclave is functioning effectively is to regularly clean and maintain it using a specialized product such as Chamber Brite.
Regular cleaning of the autoclave protects clients and staff from infection. Inadequate autoclave maintenance can cause failure or a shortened lifespan of the device and can result in your autoclave not sterilizing what it is supposed to.
Daily, monthly, and weekly cleaning of the autoclaves, strictly following the cleaning protocols given by the manufacturer, is recommended. Yearly maintenance should be done by professionals in consultation with the manufacturer.
So, what should you do if you follow all these protocols and still end up with a positive spore test result? Firstly, ensure the process indicator has not expired, and check the sterilizer for any inconsistencies. Retest and contact your biological monitoring service for assistance if the second result is positive.

