Infection control practices and sterilization standards are at the forefront of discussion as countries find themselves battling a pandemic virus sweeping the globe: COVID 19. The dental industry specifically finds itself in the frontlines for protecting their patients and staff.
A recent surge in research and innovation surrounding infection control has proven Pre & Post-Vacuum (Class B) autoclaves to be a much more effective method to match the heightened sterilization standards.
The CDC has yet to adopt the higher standards which include pre & post vacuum steam sterilization, also known as Class B sterilization. These standards have already been widely accepted in other regions of the world (Europe, Australia), and integrated in some provinces in Canada.
In this article we examine the need for the US dental sterilization method to switch to the pre-vacuum cycle/ Class B that is already being used in other parts of the world. We will also have a look at how the Tuttnauer T-Edge autoclave was designed for the current and future autoclave markets in the US.
What is a Pre & Post -Vacuum / Active air removal / Class B autoclave?
Much like the invention of the desktop computer, which gave universal access to the powers of huge data processors originally only available to research facilities, the Pre & Post -Vacuum autoclave is a miniature version of the much larger EN 285 sterilizers found in hospitals – the GOLD standard for Steam Sterilization.
Why are pre & post -vacuum autoclaves the golden standard?
The answer is – Active Air Removal!
Why is there air bad?
Effective steam sterilization is achieved only if the dry saturated steam contacts the entire surfaces to be sterilized. Nothing can come between the steam and the surface to be sterilized.
Most of the equipment we seek to sterilize contains air that acts as a barrier if it is not removed therefore compromising the sterilization process.

Gravity autoclaves vs Pre & Post-Vacuum autoclaves
The two basic types of steam sterilizers (autoclaves) are the gravity displacement autoclave and the high-speed pre & post-vacuum autoclave.
Gravity air removal principle is based on the fact that steam is lighter than air therefore it forces the air out the bottom of the chamber through the drain vent. “The gravity displacement autoclaves are primarily used to process laboratory media, water, pharmaceutical products, regulated medical waste, and nonporous articles whose surfaces have direct steam contact. For gravity displacement sterilizers the penetration time into porous items is prolonged because of incomplete air elimination.”[1]
Gravity autoclaves are the most common autoclaves in dental offices in the US since nonporous medical instruments were hardly used in the past.
As opposed to gravity sterilizers, pre & post-vacuum autoclaves remove the air from the chamber actively using a vacuum pump which enables steam penetration of hollow instruments ensuring air removal from the sterilizing chamber and load before the steam enters, therefore achieving superior sterilization outcomes.
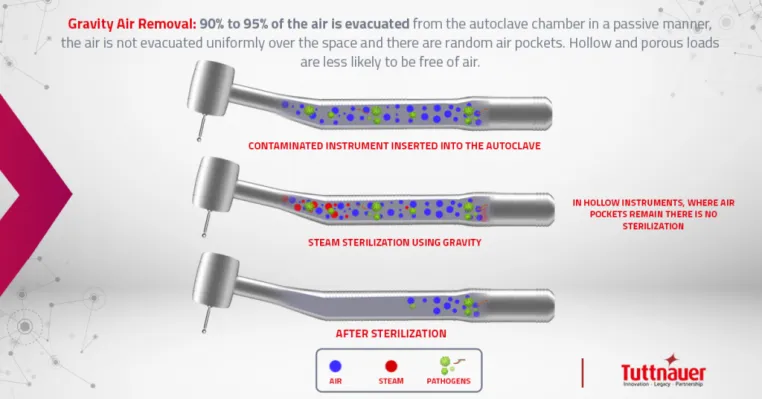
Why is there bad air during the sterilization phase?
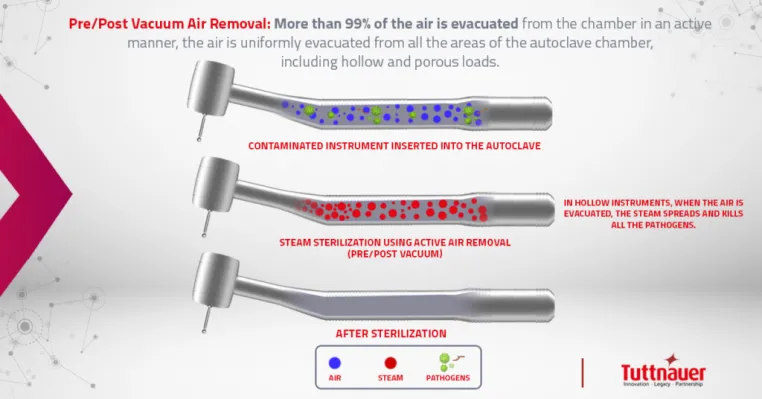
This is done in several steps.
- A few vacuum pulses remove the air inside the chamber.
- The pressure and temperature in the autoclave increase until it reaches a constant level, known as holding time. This is when the actual sterilization takes place.
- The exhaust valve releases the pressure to atmospheric pressure.
- Finally, the pressure and temperature are reduced even further to create a vacuum inside the chamber and allowing the water to evaporate into a gas, which is then sucked out by the vacuum pump!
Curious about how all this works in more detail? Checkout our blog specifically about Class B Autoclaves.
How does a non-vacuum process contribute to sterilization failure?
Sterilization failure of dental equipment can put your patients and practice at risk.
Dental handpieces present a particular sterilization concern because they have both external and internal surfaces that become contaminated during clinical use. These types of equipment can be extremely difficult to clean, inspect and sterilize because of the small size and length of lumens, intricate working parts and their inability to be readily dismantled.[2]
Gravity sterilizers use gravity displacement, which makes them less effective at penetrating hollow instruments, which have narrow lumens that can impede the ability to achieve proper sterilization conditions.
Rising concerns over infectious disease in the post-Covid-19 environment demand that regulatory bodies and dental health practitioners reexamine the current methods employed in the sterilization of dental equipment.
The solution to raising standards and improving infection control is the Class B pre & post -vacuum sterilization autoclave, the most advanced and proven effective method available on the market.
Regulation Evolution
Recent trends in the dental autoclave market show an increasing demand for pre & post -vacuum devices that have a higher standard of sterilization. Europe and Australia have been especially committed to adapting these rigorous standards.
European Standard
N class cycles (Gravity) – used for unwrapped, solid items. Steam pushes the air downwards using gravity and forces it out through the bottom of the chamber.
S class cycles – specified by the manufacturer and used with multi-pulse positive vacuum steam sterilizers to suit loads of certain types and configurations.
B class cycles – for hollow objects where the ratio of the length of the hollow portion to its diameter is more than 1:5. Air is exhausted by a mechanical pump to create a vacuum before steam is introduced into the chamber.
The European Standard is called EN 13060. Under this regulation, the performance level of small steam sterilizers – such as those used in dental practice – was elevated to medical grade. The aim of this legislation was to ensure the prevention of cross infection. **
The Australian standard is based on the European standard.
United States
Today there are strong voices calling to elevate dental instruments sterilization to medical grade (Class B, Pre & Post -Vacuum active air removal).
Tuttnauer T-Edge Autoclave
The T-Edge is Tuttnauer’s newest and most innovative tabletop autoclave that meets the increasing demand for advanced sterilization standards in dental practices and the medical industry.
T-Edge is the only gravity and airflow removal autoclave upgradable by software only to pre & post vacuum designed to meet all the most current sterilization standards ANSI/AAMI ST55.
The T-Edge space-saving tabletop autoclave is fully automated and designed with busy dental practice in mind, offering unparalleled efficiency and safety features.

Upgrade your sterilization performance with the click of a button to transform the T-Edge autoclave from a Class S gravity displacement process to a superior Class B Pre/Post Vacuum Autoclave to safely sterilize all load types.
The T-Edge’s compact space-saving design provides optimal chamber capacity, with more than 80% of the chamber available for extra-long trays.
Easy to service, simple to maintain and eco-friendly, the T-Edge will extend the life of your equipment while meeting current international standards and regulatory requirements to protect your staff and patients.
The latest edition of this standard is: EN 13060:2014+A1:2018 Small steam sterilizers