Instrument processing space & treatment room
Cluttered spaces require more time for cleaning and disinfection, as well as wasted time on searching for needed items. A clean streamlined space has a better daily flow. Efficiency not only saves time, but money as well. From an infection prevention standpoint, the Centers for Disease Control and Prevention (CDC) recommends having out only the items needed for the procedure and unit dose dispensing supplies to avoid wasted materials.1,2

Dental staff enter and exit the sterilization area multiple times per day.3 Having adequate flow in the sterilization center is critical to efficiency.3 Using a designated clean side/dirty side approach, a U shaped, or clockwise directional flow is best.3 Ensuring there is enough space for two people is also critical. Having adequate room for two people to perform processing tasks is much more efficient. A four-foot aisle way without a door is the most efficient design.4
The layout of the treatment room is an important consideration in efficiency and cost effectiveness. Treatment rooms stocked identically with color coded procedural tubs keeps staff organized, saves time trying to locate items, and reduces wasted supplies. All these considerations are effective solutions.
Labor savings
Cost effectiveness is another important metric to consider. Labor is the largest expense in a dental practice, consuming approximately 25% of the budget.3 The average dental assistant enters sterilization area up to 80 times per day to perform disinfection and sterilization tasks.3 Practices often avoid investing in sterilization equipment, mistakenly believing it saves money. Money is spent on labor instead. Investing in the right equipment can reduce labor costs. Large purchases with sterilization equipment can pay for itself within a year.
Workflow efficiencies
The flow of instrument processing can be more efficient if performed one way or can be less efficient if performed another way. The workflow begins with receiving (transporting, cleaning, and decontamination), preparation and packaging, sterilization, and storage.1 The receiving process of handling loose instruments, rinsing, and drying is very labor intensive and time consuming and puts clinicians at higher risk for percutaneous injury. This method is inefficient and unsafe. Using cassettes and an instrument washer saves valuable time and money by reducing the handling of instruments. It is much more efficient from an organization standpoint to use instrument cassettes.
Cassettes
Use of instrument managements systems (IMS) such as cassettes helps create a more organized process by easily being able to find the right instrument when needed. Cassettes help clinicians save time not having to search for loose instruments on a tray. Being unorganized costs time. Stopping to look for an instrument or supply, having to un-glove, or leave the operatory is not efficient. These little time wasters add up if done several times per day. Cassettes are also safer and reduces the risk of sharps injuries.1
Instrument Washers
The use of instrument washers or washer/disinfectors is another critical element in an efficient process. Instruments must be free of debris that inhibits the sterilization process.1,4 Washers clean, disinfect, rinse and dry instruments saving valuable time and avoiding the potential for percutaneous injury. Instruments must be thoroughly cleaned and dried prior to preparation and packaging for sterilization.1-2,4 Instrument washers eliminate several steps of manual labor creating a more efficient and safer process. The CDC recommends automated methods for cleaning.1 A good quality washer can easily handle the needs of a 4-6 operatory office. Investing in this technology can be initially expensive, but over time the return on investment in labor savings is huge. Use of cassettes along with instrument washers can save up to 10 hours of labor per week which equates to 40 hours per month in labor savings which is efficient and significantly cost effective.3
Once instruments are cleaned and dried, chemical indicators are placed to demonstrate that items have been exposed to the sterilization process.1,4 Type 5 integrating indicators are used inside cassettes and secured with a type 1 indicator tape, or pouches with type 4 indicators are used. Both methods are acceptable and are efficient.

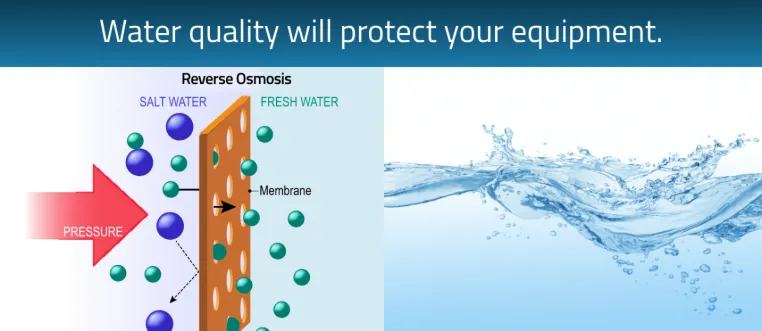
Water Quality- protecting your equipment
Water is another important consideration for the sterilization area. Good quality water is needed for the autoclave and instrument washer. Distilled water or reverse osmosis (RO) water systems provide the water quality needed for these devices. Autoclaves require either distilled or deionized (DI) water to run efficiently and reduces the risk of build-up and costly repairs. Deionized water comes from a reverse osmosis system and compares to the same quality as distilled water. Some instrument washers also use distilled water purity for the final rinse cycle. New technology now have models with an auto filler that is paired with the washer and autoclave. Using a RO system to provide DI water with autofill as opposed to the purchase of distilled water jugs is also much more efficient and cost effective. Having the RO system readily available in your central sterilization saves time, and reduces the risk of running out. Carrying and storing jugs of distilled water is time consuming and takes up space in the practice. Over time, the investment in a RO auto filler system can reap big rewards. Reverse osmosis systems are more cost effective and efficient in the long run. These are turn-key solutions for washing/disinfecting/drying and the complete sterilization cycle.
IFU – capacity to drive efficiency
Sterilization with an autoclave is used for critical and semi-critical items. It is imperative that dental staff understand the manufacturer’s instruction for use (IFU) on how to properly load, operate, unload, and monitor with biological indicators (BI).1,4 It should be noted that the sterilization capacity should match the instrument cleaning capacity. Practices often make a mistake of increasing cleaning (with a washer), but do not have the sterilization capacity to match the need. To achieve efficiency with the process, you need to match capacity. As a rule, it is good practice to have a standard autoclave with a chamber size of at least 11 inches and a flash sterilizer.3 Larger practices may need multiple autoclaves to match capacity. New autoclave technology also includes a virus protect cycle which adds another layer of safety.

Biological Monitoring
The only way to know if an autoclave is working properly is by testing or validating its effectiveness using biological monitoring, also known as spore testing.1,4 New technology includes ultra-rapid (twenty minute) biological monitoring. This method provides quicker results saving time and provides peace of mind. Keeping a log of details related to sterilization cycles is required for each load, noting the date, time, which autoclave (if there is more than one), the temperature, and duration of the cycle.1,4 Some autoclaves can be connected by USB or provide an electronic printout making logging and record keeping convenient and more efficient. In-office B.I. solutions are cost effective in the long run compared with mail-in, preventing possible contamination by using an instrument which failed a sterilization cycle, due to results wait times. Tuttnauer B.I. solutions include incubators holding several B.I. vials and save mailing costs and waiting times.

In summary, the use of cassettes, instrument washers, having adequate space/flow/capacity, being organized, use of tubs, use of RO water systems with auto fill, and use of rapid BI’s with logging capabilities all contribute to an efficient and cost-effective complete infection control solution.
References
1. Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings-2003. MMWR 2003;52(RR-17):1-66.
2. Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings. Basic Expectations for Safe Care. Available at: https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe-care2.pdf
3. Tholen, M. Increase production and decrease expenses in sterilization. Dental Economics. Oct 2009. Available at: https://www.dentaleconomics.com/practice/overhead-and-profitability/article/16389257/increase-production-and-decrease-expenses-in-sterilization
4. Occupational Safety & Health Administration. Bloodborne Pathogens Standard 29 CFR.1910.1030. Available at: https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=10051
