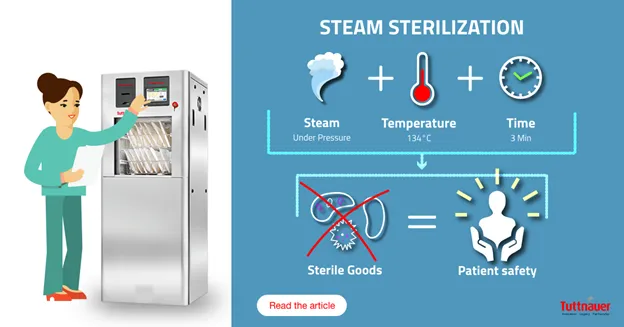
Let us begin with sterilization, steam sterilization, and their related terms.
Sterilization refers to a validated process used to render a product free of viable microorganisms, consisting of a sequence of steps designed to achieve sterilization. This is not to be confused with ‘Terminal sterilization’, in which the items are sterilized using a sterile barrier system. Steam Sterilization uses saturated steam under pressure at a specified temperature and exposure time. Validation in this context refers to the manufacturer’s documented procedure that allows results to be obtained, recorded and interpreted to check and ensure that the sterilizer will consistently meet the required specifications. Validation is performed by the manufacturer during the pre-market phase, then at the time of installation, maintenance, and periodically as defined by local requirements and the manufacturer’s instructions for use (IFU).
Read all about Sterilization Methods and the different methods of sterilization used in the medical, dental, laboratory and pharmaceutical fields here https://tuttnauer.com/blog/sterilization-methods-learning-series.
Types of sterilizers
There are questions regarding types of steam sterilizers. Gravity displacement sterilizers rely on displacement of air by gravity when steam enters the chamber, also known as Class S-type autoclaves. Steam-flush-pressure-pulse autoclaves involve air removed by a series of steam flushes and pressure pulse and is one of two types of sterilization cycles. They are not a separate category from dynamic air removal autoclaves (Class B-type). For all other dynamic air removal autoclaves, a series of pressure and vacuum excursions is used to remove air, i.e., the ‘prevacuum cycle.’ Regarding Class N-type autoclaves, their exact mode of action and parameters depend on the given manufacturer’s Class N-type autoclave. Another term we come across is ‘warm-up period’. This is only applicable to some benchtop (counter-top) steam sterilizers and occurs prior to the sterilization phase. If required, a warm-up cycle and will be indicated in the manufacturer’s IFU.
Understanding the benefits and use of each autoclave class, as well as the regulations required, will be helpful prior to purchasing an autoclave that works best for your needs. Learn more about specific details and benefits of each tabletop autoclave here https://tuttnauerusa.com/tabletop-sterilizers/markets/medical-clinics/

Mechanical, chemical and biological indicators
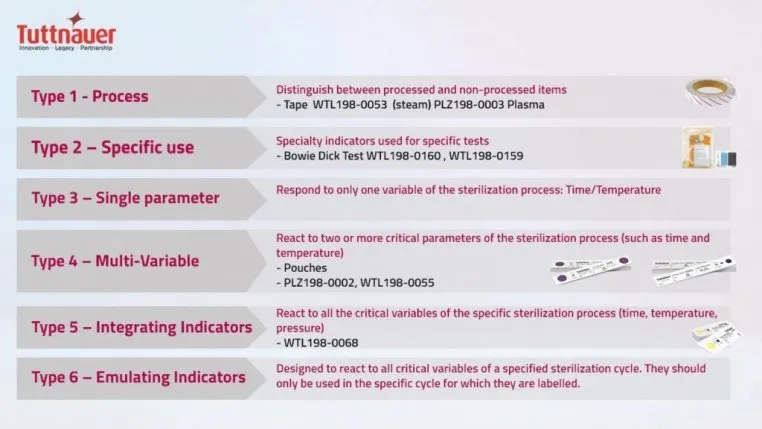
As we think about the measures taken to assess steam sterilization, these consist of mechanical, chemical and biological indicators. The mechanical indicators simply provide an analog or digital reading as to whether the parameters of sterilization – time, temperature and pressure – were reached. Chemical indicators are the second method used to monitor the sterilization process. These are designed to respond to a change in the parameters, or in the case of a Type 2 indicator to determine whether proper sterilizer functioning of pre-vacuum autoclaves and air removal was achieved. The Type 1 is an external indicator that the pack was exposed, and Types 3 through 6 variously indicate whether one or more of the parameters, a specified set of parameters or parameters for a specific cycle were achieved. They indicate potential sterilization failures, in effect an early warning system. The term Parametric Release refers to the ‘release of load based on data from the sterilization process.’ In other words, based on the results shown for indicators that all parameters in the process were met before the packs in the load were released. Parametric release is not based on sample testing, nor on the results of biological indicators (spore tests).
The use of biological indicators
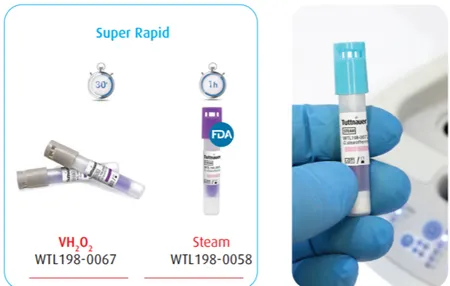
Biologic indicators consist of a standardized and viable population of spores known to be resistant to the sterilization process being monitored. For steam sterilization, Geobacillus Stearothermophilus spores are used. A spore vial or strip is placed in the autoclave during the process, and a positive control is used outside of the autoclave. This positive control is similar in concept to positive controls in clinical trials – you are comparing the test vial/strip to something intended for the same purpose. In the case of a biological indicator, the positive control vial or strip must be from the same lot of vials/strips. The term ‘positive result’ for a biological indicator can be confusing. We usually think of positive as being, well, a positive thing. For biological indicators, we want to see a negative result – that there are no spores remaining alive for the spores in the autoclave and that the positive control spores are still alive. In other words, the test passed. Biologic indicator tests show whether the conditions in the sterilizer were sufficient for sterilization. They do not prove that everything in the load is sterile or was exposed to adequate sterilization conditions. Tuttnauer manufactures super rapid biological indicators for steam sterilization, providing results in just 20 minutes, this technology allows for efficient turnaround and work flow.
For larger autoclaves, the spore test is performed using a commercially-available challenge test pack, also known as a process challenge device. The purpose of this is to mimic the situation for instruments/devices being sterilized. It consists of a test pack or tray containing the biologic indicator.
What is sterility assurance?
What about the term sterility assurance, or Sterility Assurance Level (SAL)? This is presented as a log reduction and refers to the probability of a single viable microorganisms being present after sterilization has been completed. A one in a million chance of one being present is a Log 6 reduction, noted as SAL of 10-6 which is regarded as the appropriate SAL for sterilization. The manufacturer must show that the sterilizer can provide the SAL.
Other considerations include the use of the terms ‘sterile’ and ‘sterilized’. Sterile means that the process was successful and that the item is devoid of viable microorganisms. However, there are several reasons why it may not subsequently be sterile. Firstly, was it sterilized in a sterile barrier system. The term sterile barrier system refers to a barrier that is the minimum required to prevent entry of microorganisms and allows aseptic presentation of the product at the point of use. If a sterile barrier system is used, e.g., a plastic-paper pouch or sterilization wrap, then provided the pack was dry on exit from the autoclave and remains intact until at the subsequent point-of-use, the contents (provided sterilized) are still sterile. A sterility maintenance cover, in contrast, also known as a dust cover, fits over the sterile barrier system. Depending on the regulations or recommendations in your area, it may be permissible to sterilize instruments or devices unwrapped, and to then wrap them when they exit the autoclave. In that case, after exiting the steam sterilizer they can no longer be considered sterile.

There are many other terms used in the lexicon of steam sterilization processing, however, these are some of the critical ones applicable to your day-to-day practice.
