Definition: Connectivity is defined as ‘the quality, state, or capability of being connective or connected.’
On a broad level, for example connectivity is the ability to retrieve information at a moment’s notice on the internet helps to make it easier to stay up-to-date. On a personal level, connectivity examples include monitoring babies while they sleep or houses while owners are away, and medically for robotic surgery procedures and a host of other purposes.
Protocols performed as part and parcel of infection control and prevention also derive significant benefit from connectivity. This may encompass the use of data loggers, messaging, remote monitoring or integrated documentation and digital archiving of steps in the process. In this article, we’ll look at examples in the context of steam sterilization (autoclaving).

Sterilization packs, cycles, monitoring and logs
Mechanical (physical) indicators
For autoclaving, recommendations and best practices required that all packs are labeled for each cycle and load. Sterilization monitoring must be performed routinely, to verify sterilizer efficacy, for every pack and every load in autoclaves, and as part of validation/qualification for an autoclave. This begins with mechanical (physical) indicators to verify that all three parameters (time, temperature and pressure) were met for the selected cycle. In turn, chemical indicators provide verification that one or more conditions required for sterilization were met either for a specific location or for each sterilization pack. Monitoring of mechanical and chemical indicators is used to verify the efficacy of the sterilization process. These indicators act as an early warning system and loads should be reprocessed if parameters were not met. The results of mechanical and chemical indicators are entered in the sterilization log along with the results of biological monitoring (spore testing) if that is a recommendation in your location and performed. All other required details, including the pack and load information is entered and the log is signed by the operator.
Tuttnauers’ Auto Reader allows for Rapid, Super Rapid and Ultra Rapid Biological Indicators.

Tracking reprocessed sterilization packs
The sterilization log and labeling together enable tracking/tracing of reprocessed sterilization packs, should it become necessary to remove these from inventory/storage and to reprocess them. This may occur because someone discovered a wet pack with internal moisture/water when a pack was opened to use the contents. Since the pack was wet, contamination could have occurred, and the contents will need to be reprocessed. Therefore, other packs from the load should be traced and if more packs are wet then all packs from that load must be reprocessed. Another example would be a failed spore test, requiring tracing of all loads since the last passing spore test. The ability to accurately trace and track processed packs and loads is an essential component of infection control.

Traditional processes used during autoclaving
Historically, mechanical indicators were analog gauges on the autoclave, which meant that the operator had to physically observe the gauges to verify the parameters. In addition, all pack labeling information was written manually, and a physical (paper) sterilization log was maintained by the operator and signed. Subsequently, digital ‘mechanical’ indicators were introduced in autoclaves providing a more readable option and now also include information throughout the cycle and information on potential problems and cycle/operational errors.
Connectivity Options and Innovations
Connectivity has introduced a host of options related to autoclaving. USB ports and data loggers have automated the process for information for logs, print-outs are available and information can be digitally archived. Best practices now include the use of electronic records by way of recording devices in sterilizers or an accessory device used for recordings and printouts. Options for labeling now also include barcoding with scans retained digitally. These are easily created, read and stored without the amount of effort required to manually create labeling. They also cannot be altered. Instrument tracking systems are also available that provide for traceability, and digital records are available for spore testing.
Tuttnauer autoclaves are designed with an advanced user-friendly control system for repeatable high performance. Including load tracing with barcodes. When loading the autoclave a barcode reader can be used to scan the barcodes of each load. Once the cycle starts the barcode numbers will be printed together with cycle information.

Remote real-time monitoring
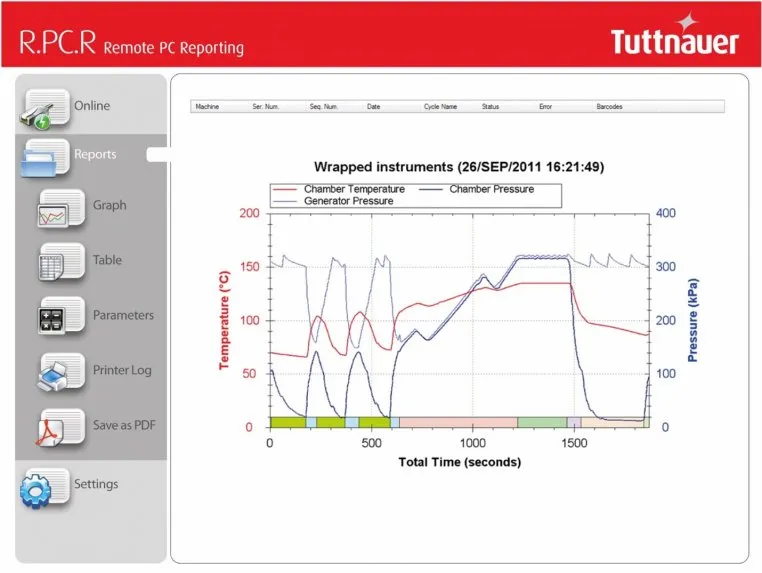
The latest innovations in connectivity for autoclaves include remote real-time monitoring of autoclave cycles, automated labeling and recordkeeping. For example, enabling autoclave monitoring remotely using a smartphone or tablet. This means there is no need to physically assess parameters at the location of the autoclave. In addition, the autoclave offers barcodes for each cycle with all sterilization data that can be affixed to reprocessed pouches and scanned for digital documentation when the contents are used for a patient. Connectivity also has provided more options for documentation since the cycle data can be printed, transferred to a PC or network through the ethernet connection, or the data can be stored in the autoclave. Remote PC Reporting (R.PC.R) Software automates and truly streamlines documentation, since all sterilization data can be saved as a pdf, transferred using a USB port or the ethernet or online, and with automated downloading of cycle information. It has become possible to digitally store information on the cycles performed, parameters reached, and to generate graphs, tables and charts for reports and analyses. It is also possible to remotely monitor multiple autoclaves, further streamlining efforts in a busy office.
Tuttnauer offers the optional addition of R.PC.R to be installed in the autoclave device. The R.PC.R Software provides cycle records you can rely on and will provide the following service:
- Automatic recording of cycle information to any PC on your network via an Ethernet network
- Convenient access to graphs and tables that are easy to understand
- Easily generate PDF reports
- No need to file printouts, saving you time
- Load Traceability - conveniently trace loads from a PC on your network with R.PC.R software. When barcodes of loads are scanned before a cycle starts, the barcode information is reported together with the cycle records. With R.PC.R you can see: Graphs of the cycle data, Numeric cycle data, Cycle print-outs, Measured values table, Parameter table.
Other Considerations
While the main focus of this article is on the contributions of connectivity to the sterilization aspects of instrument reprocessing, it is worth noting that connectivity has also enabled streamlining of protocols related to automated cleaning. For example, it is required to keep a record of all instrument washer or instrument washer-disinfector cycles, as well as testing and maintenance. Some devices have digital readouts and cycle printouts that should be reviewed for each cycle and initialed/signed by the operator to indicate an acceptable cycle. Tuttnauer Tiva washer disinfector line offers graphic reports of washing phases including time and temperature.
In Summary
As in everyday life, connectivity offers significant benefits during and after instrument reprocessing. Connectivity automates and streamlines processes and creates documentation for robust recordkeeping. Appropriate use of connectivity and recent innovations saves effort, time and standardizes digital processes with potential benefits for infection control overall.
Resources
- Association for the Advancement of Medical Instrumentation. ANSI/AAMI ST79: 2017. Comprehensive guide to steam sterilization and sterility assurance in health care facilities.
- Dubai Health Authority. Health Regulation Department. Guidelines on Dental Infection Prevention and Safety.
- Health Technical Memorandum 01-05: Decontamination in primary care dental practices. (2013)
- Koninklijke Nederlandse Maatschappij tot bevordering der Tandheelkunde. Richtlijn Infectiepreventie in Mondzorgpraktijken
- Merriam-Webster. Available at: https://www.merriam-webster.com/dictionary/connectivity#h1
