As the economy and disposable income bounces back, a rigorous safety infection prevention plan will become the new standard, not only for compliance of industry regulations but the need to reassure the public at large of dental safety. The American Dental Associationi and leading infection control researchers such as Dr. Gordon and Dr. Rella Christensen agree that dentistry must upgrade its infection control protocol in order to meet current and future global pandemics.
Modern dental care has taken its place alongside other medical specialties and dental protocols for infection control and sterilization must parallel the medical communities accepted practices.
This series will cover aspects regarding a proper sequence of chemical and biological indicators in the instrument processing.
Organizations Responsible for Sterility and Safety
The Centers for Disease Control and Prevention (CDCii), as well as the Occupational Health and Safety Administration (OSHAiii) and Bloodborne Pathogens Standard (BBP) requires verification of dental instrument sterility for the delivery of safe patient care iv, v. A proper sequence of instrument processing must be followed as outlined by the CDC including safe transportation, receiving, cleaning and decontamination, preparation and packaging, sterilization, and storage iv.
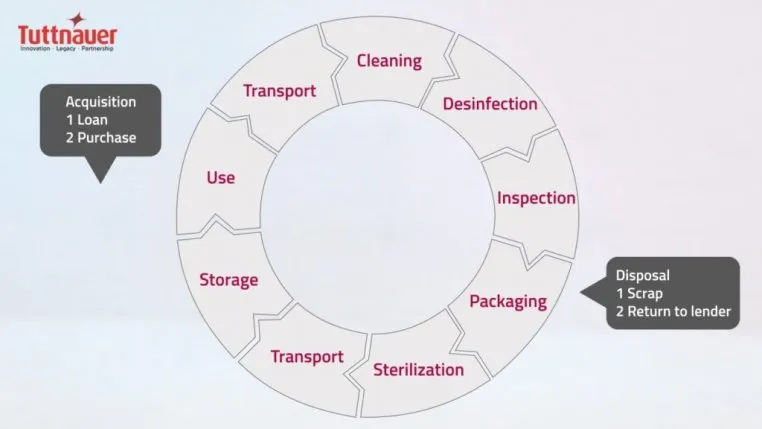
So, What Are the Regulatory Guidance for Sterilization Monitoring?
Proper sterilization monitoring processes includes a combination of mechanical, chemical (CI) and biological indicators (BI).
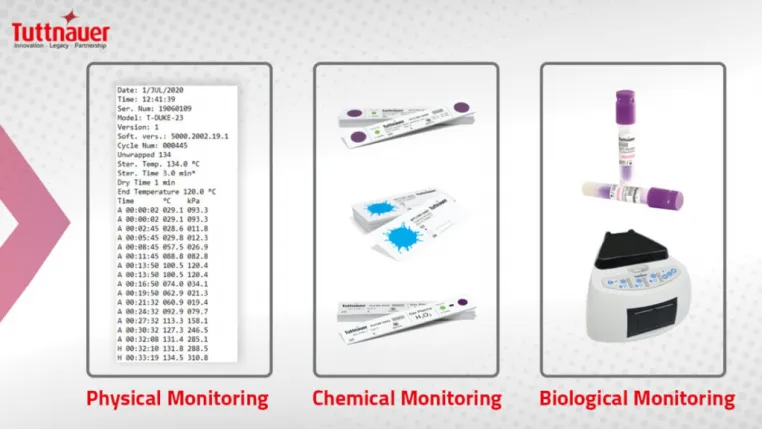
Several infection control recommendations related to CI and BI are outlined in the comprehensive 2003 CDC guidelines vi which remains the gold standard in dentistry.
The Guidebook Challenges
CDC guidance is broad and open to interpretation. There is little standardization in dentistry, as opposed to the comprehensive and strict sterilization guidance within the healthcare sector. Dental practices interpret and implement CDC recommendations in a variety of ways which is problematic especially during the COVID-19 pandemic.
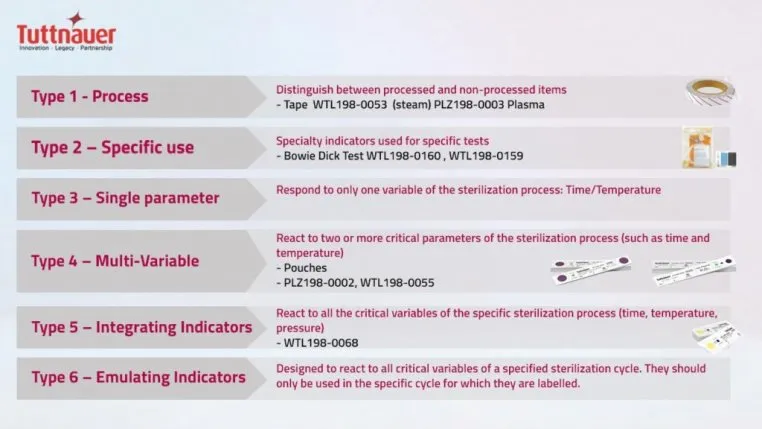
Types of Chemical Indicators
During preparation and packaging CI’s are added to monitor a variety of internal and external parameters to determine if steam has reached the contents inside.
There are six types of CI’s vii:
How to Retain Best Practices using CI
Best practices with CI’s depend upon the type of packaging used.
If pouches are used, they should be Type 4 and should be visibly labelled with non-toxic ink, on the non-porous side with the date of sterilization, the sterilizer number, and the cycle number to aid in traceability.
If cassettes are used, Type 5 integrating indicators must be used inside each cassette and wrapped using FDA approved wrap and secured with a Type 1 tape. The same labeling applies to cassettes. Bar coded labeling systems provide a much higher standard and prevent any issues with visibility and traceability. Use a clickable button/link that leads to product page.
Biological Indicators (Spore Tests)
Biological indicators (BI), also known as “spore-tests,” are used to monitor the functioning of autoclaves, by testing for the inactivation of highly resistant microorganisms. Inactivated BI indicates pathogens in the load have been killed, thus resulting in the verification of “sterilization.” Performing BI is the only way to ensure that sterility has been achieved.
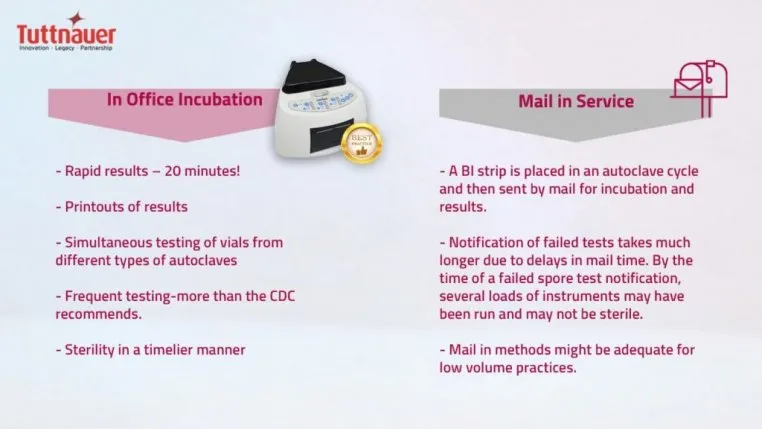
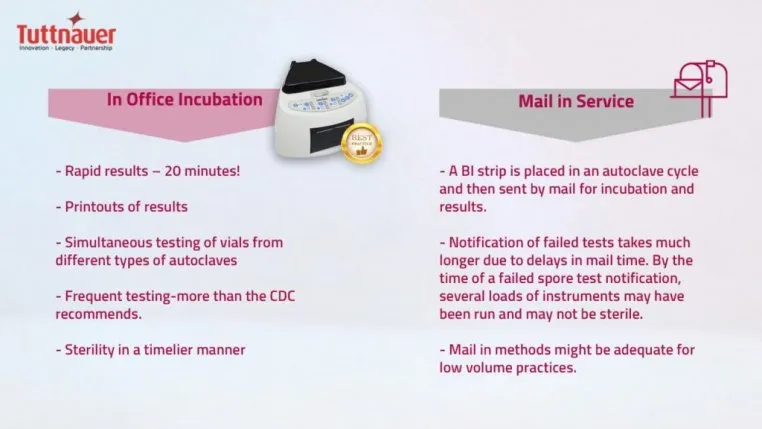
Two Methods of BI
Two acceptable methods of BI testing are available:
Biological Indicators During the COVID-19 Pandemic
Since the COVID-19 pandemic, patients and dental staff are on higher alert with infection control practices and need to always ensure sterility. In-office incubation methods of BI are preferred to ensure verification of sterility in a timelier manner (less than 24 hours). Tuttnauer cutting edge in-office BI is the fastest on the market, providing results in only 20 minutes.
The up-front cost is more expensive, however provides a rapid peace of mind. In this process a test vial is placed and run in a regular autoclave cycle, is incubated for specified time-period, and then a color change is compared. If a test fails, it is re-run. Most BI test failures are a result of operator error such as overloading and incorrect BI placement. Truly failed tests are much quicker to flag, retrieve, and log.

How often is BI recommended?
Exceeding the minimum CDC requirements is the best way to ensure sterility for patients. Currently CDC recommends performing BI at least weekly at a minimum. The risks of performing BI on a minimal weekly basis includes the potential for disease transmission and inability to retrieve potentially unsterile instruments. Tracking and retrieval could be challenging. Keep in mind CDC recommendations are minimum standards. Best practices, especially in the age of COVID-19, includes performing BI more frequently, such as daily or multiple times per day depending on the practice volume and types of loads.
The Ultra-Rapid Technology from Tuttnauer
Tuttnauer provides a full range of CI and BI products with the latest technology in BI. Two rapid in-office BI incubation models are available.
How can you achieve Overall industry Best Practices?
At the end of the day, it is all about frequency. The importance of frequent BI testing cannot be stressed enough, especially during a pandemic like COVID-19. Dental staff must ensure safe dental visits by raising minimum standards and performing best practices with infection control. Performing ultra-rapid BI testing/monitoring more frequently (daily) is one example of best practices. Various stakeholder organizations published guidance documents beginning March 2020 and continue to be updated as more research on COVID-19 evolves.
