This series will cover aspects regarding the demands of the Federal Occupational Safety and Health Administration and the sterilization equipment that Tuttnauer has to offer:
- Gravity displacement (Class N)
- Dynamic air removal (Class S and B)
- Latest sterilization technology: T-Edge
- The “Virus Protect cycle”
- T-Quick autoclave
Why is sterilization important now more than ever?
Dental professionals should be well versed in the importance of protecting patients, staff, and the public from infection. The pandemic has brought the importance of practicing good hand hygiene and the use of proper personal protective equipment into sharp focus, but it is also essential to scrutinize every aspect of the clinic. There is no better time to confirm that everyone is adhering to standard precautions, and that includes proper environmental infection control.
Who are the “shot callers” regarding infection control?
Federal Occupational Safety and Health Administration (OSHA) standards are mandated to protect the safety of workers; therefore, employers must comply or face enforcement penalties. OSHA is the regulatory body that can investigate and impose fines for lack of compliance.
Working together with the OSHA is the CDC which is an advisory body that provides evidence-based recommendations. The 2003 CDC “Guidelines for Infection Control in Dental Health-Care Settings” are the most comprehensive “rules” for dentistry.
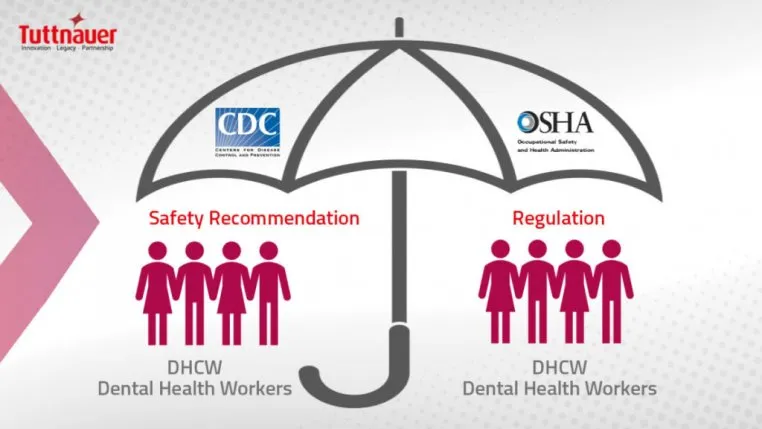
Recommendations for due to COVID-19 pandemic
Prior to the COVID-19 pandemic, DHCW was more concerned with BBP (Blood Borne Pathogens) than with respiratory pathogens. However, the outburst of the pandemic brought new recommendations for infection control.
Interim guidance from the CDC was published in response to the COVID-19 pandemic. New recommendations include social distancing, universal source control, pre-screening protocols, personal protective equipment (PPE), aerosol reduction, and environmental controls.
There has been no new guidance from CDC regarding disinfection and sterilization. As OSHA standards and CDC guidance remain the same during the COVID-19 pandemic, it is incumbent upon the DHCW to exceed minimal standards to provide an extra assurance of safety in preventing disease transmission.
The minimal criteria for sterilization of all critical and semi-critical items
The CDC requires heat sterilization for all critical and semi-critical items in compliance with the OSHA. Monitoring of the process is critical to ensure that proper sterilization parameters are reached. Use of physical (watching gauges for time, temperature, and pressure), chemical (color changes on indicators to verify all sterilization parameters are met), and biological (regular spore testing with BI) monitoring is required by the CDC. The spore testing requirement from the CDC is weekly. Keep in mind, CDC requirements are minimal standards.
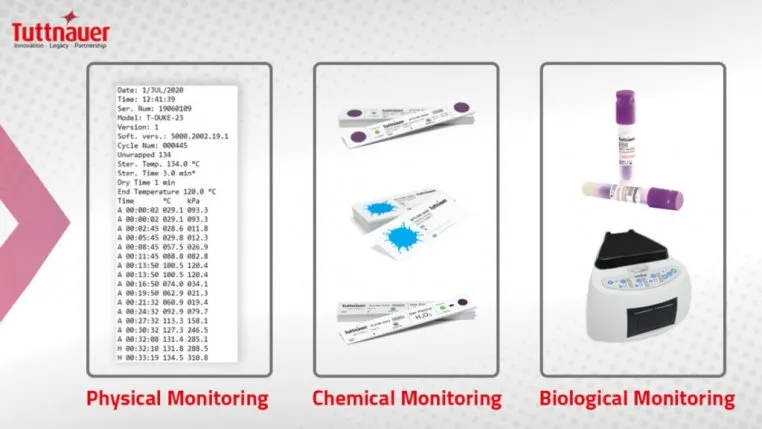
The non-minimal infection control solutions for DHCW
Tuttnauer provides infection control solutions to help clinicians exceed the minimal standards and provide the safest level of care.
Instrument reprocessing is multistep and includes cleaning (via ultrasonic, washer, or washer/disinfector including rinsing and drying), packaging (use of internal/external chemical indicators), sterilization, and storage. Tuttnauer provides solutions for all stages of the instrument reprocessing cycle from cleaning to packaging, ultra-rapid biological monitoring systems, and sterilization.
How to be one of the Best practices in the age of COVID-19

Latest sterilization technology Dynamic air removal vs. Gravity displacement
Autoclaves are FDA cleared medical devices that utilize steam under pressure and are most common in dentistry. The autoclave is an instrument responsible for the sterilization of dental instruments
There are two main types of autoclaves:
- Gravity displacement (Class N)
- Dynamic air removal (Class S and B)
Gravity displacement units are most common in dentistry. These admit air at the top or side of the chamber and since steam is lighter than air, gravity forces the heavier air out and exits through the bottom drain. This passive method requires more drying time. Trapped air or cool pockets can be problematic with Class N, so placement of the BI is critical.
Dynamic air removal sterilizers are more advanced and are the best choice for providing complete sterilization. Dynamic air removal sterilizers, such as Class S and Class B, use a vacuum pump to fully remove air from the chamber before steam is emitted. Air removal is critical for steam penetration and complete sterilization.
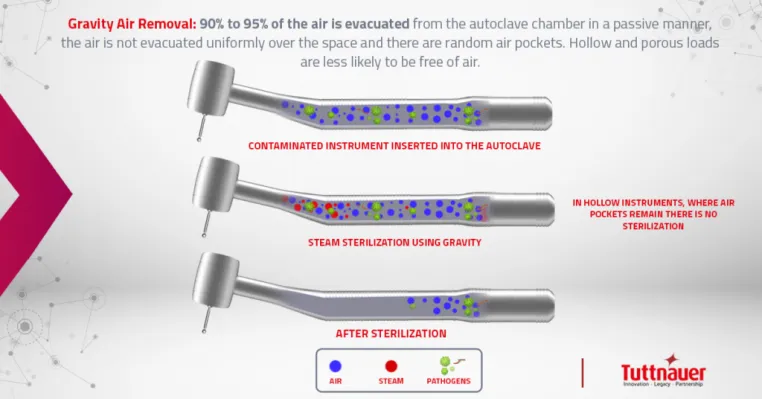
The advantages of class S and B include nearly instantaneous steam penetration, shorter drying time, and ability to fully penetrate even small hollow tubing.
No matter which type of sterilizer, it is critical to let the load cool and dry fully to avoid wicking microorganism into the contents. The strict European standard EM 13060 defines requirements for small steam sterilizers. It is widely used outside the U.S. Class B sterilizers meet this strict European standard.
Dynamic air removal autoclaves provide the highest level of sterility assurance. Tuttnauer offers a wide range of autoclaves to meet the needs of clinicians looking to enhance their infection control practices.
The latest sterilization technology from Tuttnauer is the T-Edge, the only upgradeable autoclave available on the market, which can be upgraded from a Class S to a Class B with a simple software installation. It includes a fast complete 32-minute cycle including complete closed door drying to eliminate wicking, a variety of documentation and traceability options with barcodes, and smart technology. Advanced features such as printouts, barcodes, and smart technology makes documentation easy to stay fully compliant and exceeds the CDC minimum requirements. This unique product is the only one of its kind on the market today. Utilizing a class B autoclave meets the highest standards of sterilization.
Frequent and Quick validation of the sterilization
Since the CDC requirements are minimal standards, the most stringent practices in the age of COVID-19 include performing biological monitoring (BI) daily. Tuttnauer offers 20-minute rapid “in-house” BI systems that helps clinicians exceed minimal standards.
Performing daily BI “in house” is time efficient and exceeds current standards.
Ensuring that sterilizers are functioning properly is critical for safe practice and eliminating the spread of disease
Tuttnauer “Virus Protect cycle” for COVID-19
Tuttnauer addressed the urgent needs of customers during the COVID-19 pandemic with new technology, the Virus Protect cycle. This unique cycle for class S and B autoclaves, treats/disinfects the air in the chamber before the preliminary air removal phase, ensuring that air released back into the ambient air is free from microorganisms and viruses such as Coronavirus. The cycle includes a 15-minute pre-heating stage without air venting to disinfect or treat the air before the first air removal phase. The standard sterilization cycle begins afterward.
The need for enhanced protection arose from concerns about the possibility of harmful microorganisms being released into the ambient air during the first air removal phase. Research demonstrates that SARS-CoV-2 can survive in aerosols up to a few hours. Protection from harmful pathogens became Tuttnauer’s top priority. All new units shipped from Tuttnauer have this innovative cycle. The use of Class B autoclaves with Virus Protect is the perfect solution for enhancing protection in the age of COVID-19 and exceeding CDC requirements.
The quick reprocessing of dental instruments
The T-Quick autoclave meets this need with faster cycles (25 minutes) that deliver dry instruments without wicking. This technology provides closed door drying (instruments dry without having to monitor and open the door, saving time), allowing more cycles throughout the day, and an automatic safety shut off. The T-Quick utilizes HEPA filtration that provides sterile, bacteria free air for drying. Using this technology can address capacity issues as loads can be run more frequently throughout the day. Closed door drying can further prevent accidental wicking of microorganisms into the contents. Assuring complete sterility is vital in the age of COVID-19.
Exceeding CDC recommendations is best practice in the age of COVID-19 to assure peace of mind for all.
REFERENCES
- Occupational Safety & Health Administration. Bloodborne Pathogens Standard at: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030. Accessed September 17, 2020
- Occupational Safety & Health Administration. Enforcement at: https://www.osha.gov/enforcement. Accessed September 17, 2020.
- Occupational Safety & Health Administration. Commonly Cited Standards at: https://www.osha.gov/pls/imis/citedstandard.naics?p_esize=&p_state=FEFederal&p_naics=621210. Accessed September 17, 2020.
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003; 52:1–66.
- United States Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Available at: https://www.cdc.gov/oralhealth/infectioncontrol/summary-infection-prevention-practices/index.html. Accessed September 17, 2020.
- United States Centers for Disease Control and Prevention. Guidance for Dental Settings. Interim Infection Prevention and Control Guidance for Dental Settings During the COVID-19 Response. Available at: cdc.gov/ coronavirus/ 2019-ncov/ hcp/ dental-settings.html. Accessed September 15, 2020.
- Association for the Advancement of Medical Instrumentation. Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities. Available at http://my.aami.org/aamiresources/previewfiles/1709_ST79Preview.pdf. Accessed September 15, 2020
