5th post in series, "Autoclave Sterilization Basics"
In our previous post, we explored the basic concepts of vapor, steam, boiling, evaporation, and condensation. Now let’s take a look at why steam is considered the best sterilization agent, or sterilant, for autoclaves. We will look at three main reasons: heat, pressure, and biology.
But first let’s just review what happens in the autoclave. We open the door, and place the load [1] [2] inside the autoclave chamber. The load refers to whatever we wish to sterilize, i.e., surgical instruments, as well as laboratory essentials like solids, liquids, or hollows. Once we close the door and set the autoclave to the appropriate cycle, water begins to enter the chamber and heat up to 121℃ or 134℃, thus creating steam. Steam can be produced either inside the chamber or externally by a steam generator.
Heat
Inside the autoclave, all air is removed and only steam remains. This pure steam is called saturated steam. “Saturated” means that the space occupied by the steam cannot contain any more steam, that it is completely imbued with a high level of super-concentrated steam. The optimal composition of steam within an autoclave is 3% liquid and 97% gas. Any change in the percentage of moisture increases or decreases sterilization time.
After some time boiling, the air which was in the chamber above the water will be flushed out with the steam through the vent. Then only pure steam is in the chamber. This team is called saturated steam.
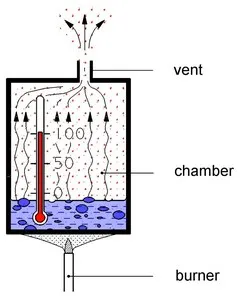
When saturated steam touches the surface of a colder object, such as the load in an autoclave, the heat is transferred from the steam to that colder object, thus heating it up. This is called condensation heat. Saturated steam used in sterilization reaches the high temperature of 121℃ or 134℃, which is so hot that when it comes into contact with the load and condenses, it transfers enough heat to kill all the microorganisms on the load. This is one of the reasons why steam is such an effective sterilization agent.
Pressure
As we just explained, saturated steam has a tremendous capacity to transfer heat into a colder load, allowing for the deep penetration of steam inside the load. Moreover, when the steam condenses on the sterilization load, the steam’s volume contracts (draws together and becomes smaller) until it becomes moisture on the load surface. As a result, more space is made available for the steam. The pressure, then, causes the steam to condense and then more steam is drawn into the autoclave chamber. This whole process causes the temperature and pressure inside the chamber to increase.
Biology
Another reason that saturated steam is the preferred sterilizing agent is that it contains the most humidity. The presence of humidity is crucial for the steam’s ability to kill microorganisms. Humid heat is more destructive than dry heat. Think of a wound caused by boiling water or water vapor versus a wound caused by touching something hot. The wound that resulted from moist heat will hurt more and go much deeper than the one caused by dry heat. Similarly, steam’s moist heat is able to strike microorganisms more deeply and effectively than dry heat, and that’s because steam can transfer heat better than hot air.
Additionally, many microorganisms contain water, and those that do are killed more easily by moist heat since the water inside of them compounds the effect of steam’s powerful moist heat transfer. And even for those microorganisms that have no moisture (like dry spores) or a very small amount of moisture, steam is still the preferred sterilant. How? The steam itself will hydrate the spore and make up for the lack of water inside it. Just as we explained above, a wound caused by boiling water or vapor will hurt more and go deeper than one caused by dry heat alone. So for spores, which lack internal moisture, steam will pack a wet punch of destruction.

Recap: The Steamy Sterilant
We applied our new understanding of steam (and specifically saturated steam) to understand why it’s the most effective agent for the sterilization of medical instruments. First we explained that steam transfers heat to the sterilization load in the autoclave by means of condensation heat. Then we explored how saturated steam condenses on a load, thereby increasing the temperature and pressure, which allows for a deeper penetration of the load. And finally we learned that most microorganisms contain water. The presence of this water actually improves steam’s killing power since moisture transfers heat energy into the microorganisms more effectively than dry heat.
Now that we understand the science behind autoclave sterilization, it’s time to learn more about the basic processes of autoclaves. Stay tuned for the next post in this series, which will take you deeper into the how’s and why’s of autoclave operation.
Comments and questions are welcome, as always, in the comment section below.
(Post based on Sterilization of Medical Supplies by Steam by Jan Huys. Image of saturated steam appears with permission from Huys.)