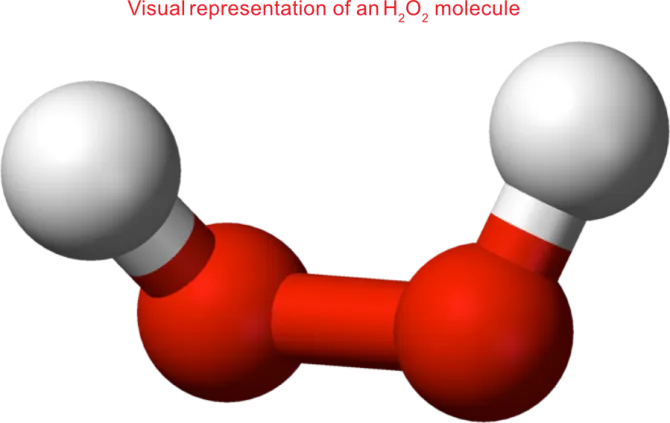
12 Questions to Answer Before Buying a Low-Temperature VHP Plasma Sterilizer
Low-temperature vaporized hydrogen peroxide (VHP) H₂O₂ plasma sterilization is the gold standard for safely and efficiently sterilizing heat- and moisture-sensitive medical devices. Operating at approximately 55°C, it delivers a 6-log reduction (SAL 10⁻⁶) against bacteria, viruses, fungi, and spores—without leaving toxic residues. Instead, it breaks down completely into water and oxygen. Leading systems like Tuttnauer PlazMax provide chamber sizes from 50 to 160 liters, load-adjusted H₂O₂ dosing, external cracking for maximum usable volume, and 1-phase power for true plug-and-play installation. This guide expands the original VHP H₂O₂ Low Temperature Sterilizer eBook with updates, deeper scientific explanations, regulatory standards, practical workflows, and a cost comparison to help you make informed decisions.
1. Why Choose VHP H₂O₂ Plasma Sterilization?
Steam is a smart, reliable way to sterilize many standard devices—but for others, it would ruin or damage them. Not all medical equipment can withstand the high pressure and temperature of an autoclave (121–134°C). The more technologically advanced an item is, the less likely it can be safely autoclaved.
Consider the classic example: an endoscope. This expensive, delicate device often includes a camera at the end of a long, flexible tube used to “scope” the body during procedures. Autoclaving an endoscope would most likely damage its optics, electronics, or seals—or, over time, destroy it completely.
As medical devices became more sophisticated and sensitive to heat and humidity, the need for a gentler alternative grew. For over twenty years, plasma sterilizers have been one of the most effective and safest solutions for low-temperature sterilization—and they remain the low-temperature sterilizer of choice in modern healthcare.
Key Benefits of VHP Plasma Sterilization:
- Operates at ~55°C—safe for delicate materials
- Achieves full sterility (SAL 10⁻⁶) against resistant spores, viruses, and fungi
- Leaves no toxic residues—decomposes to H₂O + O₂
- Fast cycles: 35–75 minutes
- Excellent penetration into lumens and complex geometries
Recognized by the CDC, FDA (Established Category A), and ISO as a proven, reliable method.
Tuttnauer PlazMax: The go-to option for endoscopy departments, ORs, and CSSDs processing high-value, heat-sensitive instruments.
2. What Is Plasma Sterilization?
Most of us learned in school that there are three states of matter: solid, liquid, and gas. But there is a fourth state: plasma.
Plasma is most similar to gas—it has no definite shape or volume—but unlike ordinary gases, plasma is highly unstable and reactive. It contains free electrons, ions, and energized particles.
A plasma is created by heating a gas or subjecting it to a strong electromagnetic field (using RF, microwave, or laser energy). This excites the molecules, strips electrons, and generates free radicals—highly reactive atoms with unpaired electrons.
Examples of Plasma in Everyday Life:
| Natural Plasmas | Man-Made Plasmas |
|---|---|
| Lightning, fire, the sun, stars, Northern Lights, auroras, >99% of the visible universe | Neon signs, fluorescent bulbs, plasma TVs, welding torches, sterilizers |
In sterilization, hydrogen peroxide (H₂O₂) vapor is ionized into plasma under vacuum. This generates hydroxyl radicals (•OH) that oxidize microbial cell walls, proteins, enzymes, and DNA—killing even the toughest pathogens.
Source: CDC Guideline for Disinfection and Sterilization
3. How Does a Plasma Sterilizer Work?
Though cycle parameters vary by manufacturer, all systems share a core process: using hydrogen peroxide as the sterilant to destroy microorganisms inside a sealed chamber. Here’s what happens in a typical 7-phase cycle:
-
Vacuum & Pre-Heat
Air is evacuated (~0 mbar), and the chamber is gently heated to ~55°C. This prevents H₂O₂ condensation on the load during diffusion. -
H₂O₂ Injection
A precise dose of liquid H₂O₂ is vaporized and introduced. Smart systems adjust dosing based on load size. -
Diffusion & Plasma Generation
Vapor and plasma pulses penetrate devices. Free radicals begin oxidation (sterilization). -
Exhaust & Cracking
Residual H₂O₂ is broken down into water and oxygen—either inside or outside the chamber. -
Repeat Pulses
Steps 1–3 repeat 2–4 times for deeper penetration and guaranteed efficacy. -
Aeration
Filtered air restores chamber conditions. -
Cycle Complete
Door unlocks—load is sterile, residue-free, and ready for immediate use.
Tuttnauer PlazMax Advantage:
- External cracking → preserves full chamber volume
- Load-adjusted H₂O₂ dosing → uses only what’s needed, saving up to 50% on consumables
4. How Is Hydrogen Peroxide Disposed Of?
After sterilization, H₂O₂ must be safely removed. There are three common methods:
- Scrubbing – Vacuum extraction + catalytic filter
- Cracking – Breaks H₂O₂ into H₂O + O₂ (non-toxic, evaporates safely)
- Both – For maximum safety
Cracking location is critical:
- Internal cracking reduces usable chamber space
- External cracking (PlazMax) keeps the full chamber available for loads
Emissions stay below 1 ppm (OSHA-compliant). No toxic byproducts. No special ventilation required.
5. Is Plasma Sterilization Safe?
Yes—and here’s why.
Plasma sterilization uses hydrogen peroxide (H₂O₂), a sterilant you likely already know from pharmacies (3% solution for wound cleaning). In sterilizers, it’s used at 50% concentration (PlazMax)—strong enough to kill microbes, but not so strong it damages devices.
The FDA recognizes H₂O₂ as a safe antimicrobial agent. After the cycle:
- All H₂O₂ is decomposed
- No chemical residues remain
- Load is safe to handle immediately
Safety Comparison: VVH Plasma vs. EtO vs. Formaldehyde
| Safety Factor | H₂O₂ Plasma | Ethylene Oxide (EtO) | Formaldehyde |
|---|---|---|---|
| Chemical residues | None | Yes | Yes |
| Safe to handle | Yes | No | No |
| Safe for environment | Yes | No | No |
| Short aeration time | Yes | No | No |
| Carcinogenic to humans | No | Yes | Yes |
| Flammable | No | Yes | No |
| Toxic gas | No | Yes | Yes |
PlazMax uses 50% H₂O₂—proven effective, device-friendly. Competitors often use >59%, increasing material stress.
6. What Size Plasma Sterilizer Do You Need?
Plasma sterilizers range from 30L to 200L+. Choosing the right size depends on your workflow.
Tuttnauer PlazMax Chamber Options:
| Model | Volume | Internal Dimensions (mm) |
|---|---|---|
| P50 | 47L | 624 × 420 × 180 |
| P110 | 109L | 624 × 420 × 420 |
| P160 | 162L | 924 × 420 × 420 |
Three Key Questions to Ask:
-
What is your typical load?
How many lumened (e.g., endoscopes) and non-lumened devices do you process per cycle? Check manufacturer load capacity charts. -
How much space do you have?
PlazMax units are narrow, mobile, and fit through standard hospital doors. Larger chambers may share the same footprint. -
Do you need loading aids?
High-volume departments use containers and transfer carriages. Ensure chamber depth allows easy access for loading, unloading, and service.
7. Utility & Installation Considerations
Unlike steam autoclaves, which require steam lines, water, drains, and vents, plasma sterilizers are true “plug-and-play” machines.
Utilities Required:
- Only electricity
- No water, drain, or ventilation
Power Options:
- Some brands require 3-phase power (costly, complex)
- PlazMax uses 1-phase → lower energy cost, easier setup
Installation:
- Arrives on wheels
- Rolls through standard doors
- Plug in and run—no construction needed
vs. Autoclaves: Save $10,000–$30,000 in infrastructure
8. What Consumables Are Needed?
Here is the complete list of consumables:
- H₂O₂ sterilant (bottles or cartridges)
- HEPA filters
- Tyvek pouches/wraps
- Chemical indicators (CIs)
- Biological indicators (BIs) + incubators
By far, the most important consumable, both in terms of functionality and cost, is the hydrogen peroxide sterilant. Manufacturers supply the H202 sterilant in cassettes, bottles, or cartridges. Just like when you buy laundry detergent, you want to know which detergent is most economical based on how many cycles you can get out of one bottle, so too, it’s important to research how many cycles your sterilizer will run per cassette/bottle/cartridge of hydrogen peroxide sterilant. The amount of H202 used per cycle depends on the size of the chamber, type and size of the load. Since the sterilant is the major consumable, it is also the most important one to investigate in terms of total cost of ownership.
The #1 Cost Driver: H₂O₂ Sterilant
- Fixed-dose systems waste H₂O₂ on small loads
- PlazMax adjusts dose by load → savings
The rest of the consumables are secondary and, depending on the sterilizer manufacturer, may be purchased from third party distributors, or from the manufacturer itself. It is also worthwhile to look into the economics here of who will be your main supplier of consumables, and how much you expect your annual consumable costs to be.
Flexibility - Tuttnauer supplies indicators and pouches.**
9. Why does Tuttnauer use only 50% concentration of H2O2 while other manufacturers use a higher concentration?
Tuttnauer’s PlazMax sterilizer agent uses a 50% concentration of hydrogen peroxide (H2O2).
This concentration is strong enough to act as an effective sterilant, but not too strong to damage endoscopes and other flexible heat-sensitive equipment. This is Tuttnauer’s advantage by using a low concentration of (H2O2) in the chamber, so that the load is not significantly affected by the sterilant. Other manufacturers work with cassettes or cartridges. These two are physically small and therefore contain a low volume of liquid. The Hydrogen Peroxide consumption differs by the volume, program and load. By using a cassette or cartridge you will not have the adequate quantity of H2O2 for a proper sterilizing process, therefore, the low volume of liquid dictates the necessity of using a higher concentration for having the same effect of sterilization. In this case, the less concentration of H2O2, the more prolonged the endoscope’s longevity. Less is more! For instance, think about if you had used the same quantity of water in a normal steam sterilizer, regardless the chamber size, the program and the load, would you have received the same results?
10. How to Ensure Effective Sterilization & H₂O₂ Penetration?
You need daily confidence that your sterilizer is working. Two essential tests:
-
Leak Test
Checks chamber seal and vacuum pump performance.The Leak Test detects if the chamber is totally sealed and that there are no leaks from outside the machine to the inside. In addition the performance of the vacuum pump is checked during this test.
-
Penetration Test
Uses a Process Challenge Device (PCD) with a chemical indicator inside simulated lumens.
The Penetration Test is a quick test to run on the sterilizer first thing in the morning to make sure that conditions for sterilization are being met. This will be verified by a chemical indicator, which shows whether sterilization has been successful. Some manufacturers provide a Process Challenge Device (PCD), which is a kit that includes lumens, connected to a cavity designed to hold an indicator. Successful penetration will be verified by the indicator result.
11. Sterilization in a PCD of 4 meters length works also in other plasma sterilizer manufacturers even though they say the longest tube is 1.4m. Why is that?
Why Tuttnauer’s PCD Kit Stands Out
Most manufacturers validate with short, simple lumens (e.g., 1–2m, one-sided).
Tuttnauer’s PCD is far more challenging—and therefore more reliable:
It is a common belief that the longer the tube is, the more challenging it can be. This is a frequent misconception.
When dealing with such a test, the length is not the critical factor, but the opening setting dictates the challenge scale. When the tube has oth sides open (two-way tube), it serves as a straw, suction forces act from both sides and makes it very easy to vacuum all air out along its entire length.
When the tube is blocked on one side, suction forces act from the open side only, therefore, a powerful vacuum is required to remove air from the far dead end.
When running a PCD test, using the 1.4m with one side blocked, it provides an ultimate indication of entire air removal and ensures maximum vaporized hydrogen peroxide spreading into the entire volume. This is the true challenge.
Why This Matters:
If PlazMax sterilizes the PCD (worst-case scenario), you can be 100% confident it sterilizes your actual instruments—even long, narrow endoscopes.
| Feature | Tuttnauer PCD | Typical Competitor |
|---|---|---|
| Lumen Diameter | 1 mm | 1–2 mm |
| Bidirectional Length | 4 meters | 1–2 meters |
| One-Sided Length | 1.4 meters | Shorter |
| Difficulty | Harder than real loads | Easier |
Compliant with ISO 11140, ISO 14937, AAMI ST58.
The PCD kit for PlazMax is a hardware tool without a predefined shelf life. When working according to the operation manual, you can use this PCD as long as it undamaged.
12. Are There Equipment Tracing Records for Endoscopes?
Yes—and it’s essential.
Endoscopes have a manufacturer-defined cycle limit (e.g., 500–1000 cycles). Exceeding this voids warranties and risks damage.
Manual tracking is error-prone.
PlazMax offers built-in digital traceability:
- Scan endoscope unique ID barcode at cycle start
- System logs every cycle per device
- Generates audit-ready reports
- Integrates with hospital IT systems
Prevents overuse. Ensures compliance. Extends device life.
13. Cost Comparison: VHP Plasma vs. Other Methods
Cost amounts mention below may change depending location or specific circumstances, and are provided for comparative purpose only.
| Cost Factor | VHP H₂O₂ Plasma (PlazMax) | Steam Autoclave (Large Horizontal) | EtO Gas |
|---|---|---|---|
| Cycle Time | 35–75 min | 15–30 min | 12+ hrs |
| Consumables per Cycle | $5–15 (load-adjusted) | <$1 | $20–50 |
| H₂O₂ Savings (vs. Fixed Dose) | Up to 50% | N/A | N/A |
| Annual Consumables (500 cycles) | $2,500–7,500 | <$500 | $10,000–25,000 |
| Installation | Plug-and-play | Piping, drain, vent | Vent, scrubber, permits |
| Installation Cost | <$5,000 | $10,000–30,000 | $50,000+ |
| Utility Cost (annual) | Low (1-phase) | High (steam) | High (gas, aeration) |
| Device Compatibility | Delicate, lumened | Heat-tolerant only | Broad |
| ROI Timeline | 1–2 years | 1–3 years | 3–5+ years |
| Regulatory Burden | Low | Low | EPA permits, monitoring |
PlazMax Delivers Lowest TCO: Efficiency + no infrastructure = rapid ROI.
14. Compatible & Incompatible Materials
Commonly Sterilized:
- Metals: Stainless steel, aluminum (6000 series), titanium
- Polymers: PTFE (Teflon), silicone, polycarbonate, nylon, PVC, polysulfone
- Devices: Endoscopes, cameras, shavers, optics, defibrillators, robotic instruments
Cannot Be Sterilized:
- Cellulose, paper, wood, linen
- Liquids, powders, oils
- Lumens <1 mm diameter or >10 m length
15. Standards & Validation
- FDA: Established Category A
- ISO 22441:2022: VHP plasma standard
- ISO 17665-1: Moist heat & low-temp validation
- ISO 11140: Chemical indicators
- AAMI ST58: Indicator requirements
Validation Process (IQ/OQ/PQ):
Includes worst-case PCD testing, biological indicators, and documentation.
FAQs on VHP H₂O₂ Plasma Sterilization
-
Why can’t I use steam on endoscopes?
High heat and moisture damage optics, seals, and electronics. -
How long is a typical cycle?
35–75 minutes, depending on load and chamber size. -
Is any H₂O₂ left on instruments?
No—fully decomposed into water and oxygen. -
Can PlazMax sterilize long lumens?
Yes—validated up to 4m bidirectional via PCD. -
Internal vs. external cracking?
External (PlazMax) = more usable chamber space. -
Does PlazMax need 3-phase power?
No—1-phase, lower cost and easier install. -
Can I use third-party consumables?
Yes for indicators/pouches; H₂O₂ from Tuttnauer only. -
How often should I run a PCD test?
Daily for penetration; full validation per protocol. -
Is plasma safe for staff?
Yes—<1 ppm emissions, interlocks, remote monitoring. -
Can I move the unit between rooms?
Yes—on wheels, plug-and-play. -
What’s the ROI?
1–2 years via high throughput and low consumables. -
Effective against prions?
No—requires alkaline cleaning or incineration. -
Can I sterilize N95 masks?
Yes—up to 50+ per cycle in larger models (validated). -
Does it integrate with hospital systems?
Yes—cycle data, traceability, IT export. -
What maintenance is required?
Quarterly HEPA filters; annual preventive service.
Tuttnauer PlazMax Sterilizer
PlazMax Delivers Unmatched Value:
- 50–160L chambers with full usability
- External cracking → no wasted space
- Load-adjusted H₂O₂ → lowest consumable cost
- 1-phase power → plug-and-play
- Endoscope traceability → compliance & longevity
- 4m PCD validation → proven penetration
- Lowest total cost of ownership
 Expanded & modernized from the 2021 PlazMax VHP H2O2 Low Temperature Sterilizer eBook — deeper, clearer, and fully verified. 🚀
Expanded & modernized from the 2021 PlazMax VHP H2O2 Low Temperature Sterilizer eBook — deeper, clearer, and fully verified. 🚀