In this blog post we will dive into the lake of lab liquids for autoclaves, also known as steam sterilizers. If you missed our last blog post in which we introduced lab autoclaves and how to process solids/hollows and isothermals, check out Sterilization of Solids/Hollows & Isothermal Processing.
Liquids
Autoclaving liquids presents some unique challenges. Liquids take longer to sterilize than other media since liquids have a high heat capacity. As opposed to solids, liquids take a lot longer to heat up and cool down, and the total cycle time is increased dramatically as a result.
With liquids, there is no requirement for air removal from the chamber in order to ensure successful sterilization whereas with solids, we do need to remove all air due to possible hollow spaces inside a load. In the case of solids, steam is the sterilization medium and must penetrate inside the load and make contact with all surfaces, which is why all air must be removed. If air were to remain inside the load (think, inside a tall beaker or flask), that would compromise steam’s ability to reach all surfaces of the load.
Liquids, however, which have no hollow spaces, use steam as a medium to heat up the liquid, but the actual sterilization takes place by the liquid sterilizing itself due to its increased temperature. Therefore, there is no requirement to remove chamber air.
In addition, due to the delicate nature of rapidly heating and cooling liquids, special precautions must be taken to avoid their boiling over inside the autoclave. The boil-over effect, as it’s commonly known, is problematic because it causes the loss of liquids and because it creates a mess inside the autoclave. Obviously, no researcher enjoys cleaning up splattered hot liquid or exploded glassware at the end of a liquid cycle, which are both unpleasant and potentially dangerous.
Liquid Cooling
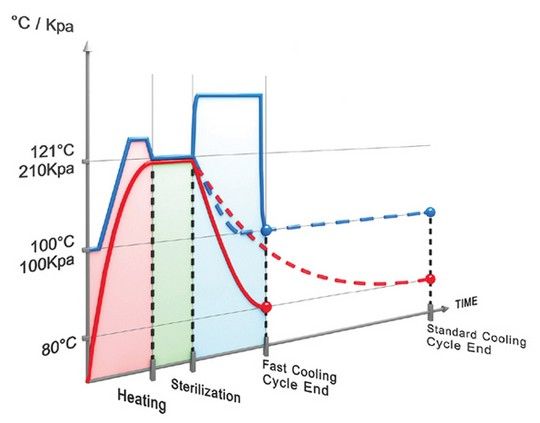
Reducing the cooling times for liquid sterilization is of paramount importance not only to save time, but more importantly, to protect the integrity of the load and ensure the liquids are not “overcooked” (except for water, which cannot be overcooked). There are two cooling applications available for liquid cooling:
- Fast Liquid Cooling will cool down the load 75% faster than leaving the load to cool under ambient (regular room temperature) conditions. After the sterilization phase is complete, chamber pressure is increased by forcing compressed air through a microbiological filter into the chamber. This increase in pressure prevents boil-over, spills and cracks in containers that would normally occur under low-pressure conditions. Since the pressure is increased, the temperature can then be safely reduced. Cold water is circulated through the chamber jacket to cool the chamber and load [link anchor to large autoclave article section about jackets]. This combination of introducing cold water and pressurized air helps reduce the temperature of the liquid load quickly and safely.
- Super Fast Liquid Cooling In addition to the introduction of high-pressure air inside the chamber and cold water circulating in the walls of the chamber, a fan can be used to further accelerate cooling by speeding up the circulation of air, which transfers the heat from the load to the cold chamber walls more efficiently. Super fast liquid cooling can reduce liquid cycle times by as much as 90%.
Two Temperature Sensors
The temperature in the chamber can reach the sterilization temperature of 121℃ way before the liquid inside the container will reach the same temperature. And the reverse is true as well: the chamber cools down much faster than the liquid load. In order to account for this disparity, we use two flexible temperature sensors, which are placed inside the liquid load, to give an accurate reading of the temperature of the liquid, thus allowing us to know when the liquid’s temperature actually reaches the proper level for sterilization. The two flexible temperature sensors are also used to control the conditions inside the chamber during the cooling phase. As a result, we are able to avoid dangerous messes caused by boil-over, and ensure safe conditions for opening the door. The reason we use two temperature sensors (in different vessels) is for safety. Should anything happen to one of them, for example container breakage, then there will be a difference between the two readings, thus prompting the autoclave operator to halt the cycle and keep the door locked until the temperature and pressure inside the chamber return to safe conditions for opening the door.

Fo Feature for Heat-Sensitive Liquid Media
One last point about liquid sterilization. Laboratory autoclaves are designed to be able to perform the Fo feature (pronounced F-zero). This is a feature that reduces exposure time of the load to high temperatures, in this case delicate heat-sensitive liquid media. In minimizing the overall time that liquid media are exposed to high temperatures, the integrity of the load is maintained, as well as reducing overall cycle time and energy costs for the lab. It allows the autoclave operator to consider the heat energy contributed during the heat-up time as contributing to sterilization. This is calculated by empirically tested tables of Fo values.
For example, the Fo feature is useful for a very delicate liquid, which can be easily caramelized and therefore disqualified for use. The autoclave operator can calculate a lower cycle time for sterilizing the liquid based on the Fo empirical tables. If a normal sterilization holding time is set to 15 minutes, it may be reduced by seconds/minutes based on the Fo calculation. This will help prevent overcooking the liquid while still ensuring proper sterilization.
For those interested in the science behind this concept, here is the actual equation that is used to calculate Fo times:
Fo = ΔtΣ10(T-121)/Z Fo values are calculated using this equation. Δt is the time interval between Fo measurements. T is the load temperature at time t and Z = 10oC. The Fo is a cumulative term, as represented by the Σ in the equation, which is determined from measurements of the load temperature (T) for the period of the sterilization process.
Fluid Sterilization
We discussed the unique challenges of autoclaving liquids, foremost among them preventing the boil-over effect. In order to achieve this, we investigated fast liquid cooling and superfast cooling, which lower overall cycle time for processing liquids. We then looked at how the two temperature sensors prevent dangerous spills and ensure proper liquid sterilization. Finally, we explored the Fo feature for heat-sensitive liquid media, which maintains the integrity of the load during processing and saves precious processing time and energy costs.
Stay tuned for our next blog post in which we will explore basic features of laboratory autoclaves.
1 In some benchtop lab autoclaves a jacket is not used for cooling, but rather two cooling pipes inside the chamber are used. Water is pumped through the cooling pipes to cool down the chamber.
